Abstract
Infiltration of the thyroid gland by lymphocytes is a hall-mark of autoimmune thyroid disease; it is particularly evident in Hashimoto's thyroiditis but is also seen in most patients with Graves' disease. Infiltrating cells are comprised primarily of T lymphocytes, of which only a minority appears to be activated. Their precise pathogenic role is largely unknown. Since perforin has been a marker for functionally activated cytotoxic T cells in situ we elected to assess the presence of perforin-containing cells in thyroid-infiltrating lymphocytes and establish their phenotype. Cells were isolated from seven subtotal thyroidectomy specimens, five from patients with Graves' disease and two with Hashimoto's thyroiditis. The novel findings were as follows: CD4+ perforin-containing T cells occurred only in Hashimoto's glands, suggesting a class II-restricted component of cytotoxicity; in Graves' disease, and to a lesser extent in Hashimoto's, perforin-expressing cells were primarily T cell receptor alpha beta- CD4-CD8- (double negative); double negative perforin-containing cells in peripheral blood of normal individuals were largely gamma delta + T cells. In Hashimoto's samples, the predominant population of T cells expressing perforin was CD8+. By comparison, in studies of the synovial fluid of knee joints from patients with rheumatoid arthritis only a minor population of the perforin-containing cells was double-negative. The data suggest significant differences in cytotoxic autoimmune mechanisms between the two autoimmune thyroid diseases. Functional characterization of double-negative T cells is necessary to define their role in autoimmunity.
Full text
PDF

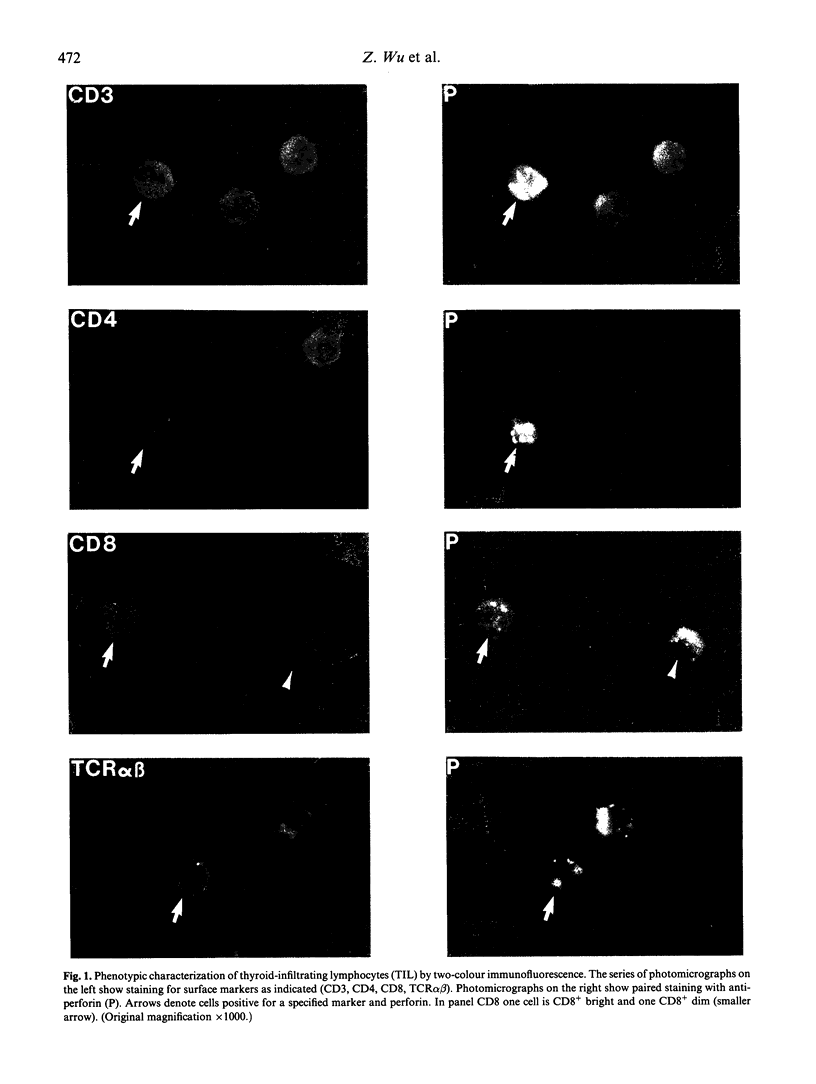

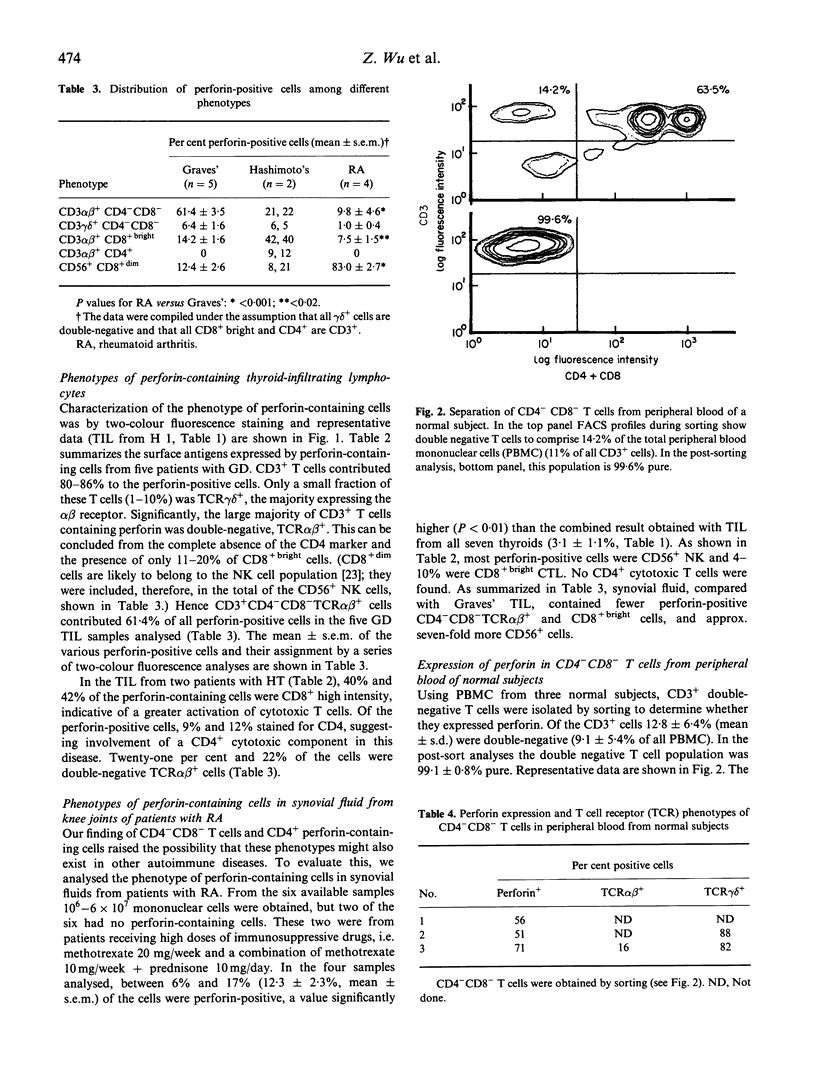



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T. Extrathymic pathways of T-cell differentiation: a primitive and fundamental immune system. Microbiol Immunol. 1993;37(4):247–258. doi: 10.1111/j.1348-0421.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Amano T., Katagiri S., Tominaga N., Oritani K., Tamaki T., Kanayama Y., Yonezawa T., Tarui S. Growth inhibition of RPMI 8226 human myeloma cells by peripheral blood lymphocytes. Acta Haematol. 1992;87(1-2):37–44. doi: 10.1159/000204711. [DOI] [PubMed] [Google Scholar]
- Bene M. C., Derennes V., Faure G., Thomas J. L., Duheille J., Leclere J. Graves' disease: in situ localization of lymphoid T cell subpopulations. Clin Exp Immunol. 1983 May;52(2):311–316. [PMC free article] [PubMed] [Google Scholar]
- Ciampolillo A., Napolitano G., Mirakian R., Miyasaki A., Giorgino R., Bottazzo G. F. Intercellular adhesion molecule-1 (ICAM-1) in Graves' disease: contrast between in vivo and in vitro results. Clin Exp Immunol. 1993 Dec;94(3):478–485. doi: 10.1111/j.1365-2249.1993.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. The lpr and gld genes in systemic autoimmunity: life and death in the Fas lane. Immunol Today. 1992 Nov;13(11):427–428. doi: 10.1016/0167-5699(92)90066-G. [DOI] [PubMed] [Google Scholar]
- Dayan C. M., Londei M., Corcoran A. E., Grubeck-Loebenstein B., James R. F., Rapoport B., Feldmann M. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7415–7419. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot L. J., Quintans J. The causes of autoimmune thyroid disease. Endocr Rev. 1989 Nov;10(4):537–562. doi: 10.1210/edrv-10-4-537. [DOI] [PubMed] [Google Scholar]
- Griffiths G. M., Alpert S., Lambert E., McGuire J., Weissman I. L. Perforin and granzyme A expression identifying cytolytic lymphocytes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):549–553. doi: 10.1073/pnas.89.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Mueller C. Expression of perforin and granzymes in vivo: potential diagnostic markers for activated cytotoxic cells. Immunol Today. 1991 Nov;12(11):415–419. doi: 10.1016/0167-5699(91)90145-J. [DOI] [PubMed] [Google Scholar]
- Griffiths G. M., Namikawa R., Mueller C., Liu C. C., Young J. D., Billingham M., Weissman I. Granzyme A and perforin as markers for rejection in cardiac transplantation. Eur J Immunol. 1991 Mar;21(3):687–693. doi: 10.1002/eji.1830210322. [DOI] [PubMed] [Google Scholar]
- Groh V., Fabbi M., Hochstenbach F., Maziarz R. T., Strominger J. L. Double-negative (CD4-CD8-) lymphocytes bearing T-cell receptor alpha and beta chains in normal human skin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5059–5063. doi: 10.1073/pnas.86.13.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Buchan G., Chantry D., Kassal H., Londei M., Pirich K., Barrett K., Turner M., Waldhausl W., Feldmann M. Analysis of intrathyroidal cytokine production in thyroid autoimmune disease: thyroid follicular cells produce interleukin-1 alpha and interleukin-6. Clin Exp Immunol. 1989 Sep;77(3):324–330. [PMC free article] [PubMed] [Google Scholar]
- Hameed A., Olsen K. J., Cheng L., Fox W. M., 3rd, Hruban R. H., Podack E. R. Immunohistochemical identification of cytotoxic lymphocytes using human perforin monoclonal antibody. Am J Pathol. 1992 May;140(5):1025–1030. [PMC free article] [PubMed] [Google Scholar]
- Hammond D. M., Nagarkatti P. S., Goté L. R., Seth A., Hassuneh M. R., Nagarkatti M. Double-negative T cells from MRL-lpr/lpr mice mediate cytolytic activity when triggered through adhesion molecules and constitutively express perforin gene. J Exp Med. 1993 Dec 1;178(6):2225–2230. doi: 10.1084/jem.178.6.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa N., Eguchi K., Otsubo T., Ueki Y., Fukuda T., Tezuka H., Matsunaga M., Kawabe Y., Shimomura C., Izumi M. Reduction in the suppressor-inducer T cell subset and increase in the helper T cell subset in thyroid tissue from patients with Graves' disease. J Clin Endocrinol Metab. 1987 Jul;65(1):17–23. doi: 10.1210/jcem-65-1-17. [DOI] [PubMed] [Google Scholar]
- Iwatani Y., Hidaka Y., Matsuzuka F., Kuma K., Amino N. Intrathyroidal lymphocyte subsets, including unusual CD4+ CD8+ cells and CD3loTCR alpha beta lo/-CD4-CD8- cells, in autoimmune thyroid disease. Clin Exp Immunol. 1993 Sep;93(3):430–436. doi: 10.1111/j.1365-2249.1993.tb08196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson R., Karlsson A., Forsum U. Intrathyroidal HLA-DR expression and T lymphocyte phenotypes in Graves' thyrotoxicosis, Hashimoto's thyroiditis and nodular colloid goitre. Clin Exp Immunol. 1984 Nov;58(2):264–272. [PMC free article] [PubMed] [Google Scholar]
- Kawakami A., Eguchi K., Matsunaga M., Tezuka H., Ueki Y., Shimomura C., Otsubo T., Nakao H., Migita K., Ishikawa N. CD4+CD45RA+ cells (suppressor-inducer T cells) in thyroid tissue from patients with Graves' disease. Acta Endocrinol (Copenh) 1991 Dec;125(6):687–693. doi: 10.1530/acta.0.1250687. [DOI] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Levis S., Ghandur-Mnaymneh L., McKenzie J. M., Zakarija M. Evaluation of the biological significance of leukocyte infiltration of the thyroid in Graves' disease. Int Arch Allergy Immunol. 1992;99(1):37–43. doi: 10.1159/000236333. [DOI] [PubMed] [Google Scholar]
- Lichtenheld M. G., Olsen K. J., Lu P., Lowrey D. M., Hameed A., Hengartner H., Podack E. R. Structure and function of human perforin. Nature. 1988 Sep 29;335(6189):448–451. doi: 10.1038/335448a0. [DOI] [PubMed] [Google Scholar]
- Lichtenheld M. G., Podack E. R. Structure and function of the murine perforin promoter and upstream region. Reciprocal gene activation or silencing in perforin positive and negative cells. J Immunol. 1992 Oct 15;149(8):2619–2626. [PubMed] [Google Scholar]
- Lu P., Garcia-Sanz J. A., Lichtenheld M. G., Podack E. R. Perforin expression in human peripheral blood mononuclear cells. Definition of an IL-2-independent pathway of perforin induction in CD8+ T cells. J Immunol. 1992 Jun 1;148(11):3354–3360. [PubMed] [Google Scholar]
- Lucas-Martin A., Foz-Sala M., Todd I., Bottazzo G. F., Pujol-Borrell R. Occurrence of thyrocyte HLA class II expression in a wide variety of thyroid diseases: relationship with lymphocytic infiltration and thyroid autoantibodies. J Clin Endocrinol Metab. 1988 Feb;66(2):367–375. doi: 10.1210/jcem-66-2-367. [DOI] [PubMed] [Google Scholar]
- MacKenzie W. A., Schwartz A. E., Friedman E. W., Davies T. F. Intrathyroidal T cell clones from patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1987 Apr;64(4):818–824. doi: 10.1210/jcem-64-4-818. [DOI] [PubMed] [Google Scholar]
- Margolick J. B., Hsu S. M., Volkman D. J., Burman K. D., Fauci A. S. Immunohistochemical characterization of intrathyroid lymphocytes in Graves' disease. Interstitial and intraepithelial populations. Am J Med. 1984 May;76(5):815–821. doi: 10.1016/0002-9343(84)90992-6. [DOI] [PubMed] [Google Scholar]
- Martin A., Goldsmith N. K., Friedman E. W., Schwartz A. E., Davies T. F., Roman S. H. Intrathyroidal accumulation of T cell phenotypes in autoimmune thyroid disease. Autoimmunity. 1990;6(4):269–281. doi: 10.3109/08916939008998419. [DOI] [PubMed] [Google Scholar]
- McGregor A. M. Autoimmunity in the thyroid--can the molecular revolution contribute to our understanding? Q J Med. 1992 Jan;82(297):1–13. [PubMed] [Google Scholar]
- McIntosh R. S., Tandon N., Pickerill A. P., Davies R., Barnett D., Weetman A. P. The gamma delta T cell repertoire in Graves' disease and multinodular goitre. Clin Exp Immunol. 1993 Dec;94(3):473–477. doi: 10.1111/j.1365-2249.1993.tb08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Pegg C. A., Atherton M. C., Middleton S. L., Dickinson A., Clark F., Proctor S. J., Proud G., Rees Smith B. Subpopulations of thyroid autoantibody secreting lymphocytes in Graves' and Hashimoto thyroid glands. Clin Exp Immunol. 1986 Aug;65(2):319–328. [PMC free article] [PubMed] [Google Scholar]
- Mieno M., Suto R., Obata Y., Udono H., Takahashi T., Shiku H., Nakayama E. CD4-CD8- T cell receptor alpha beta T cells: generation of an in vitro major histocompatibility complex class I specific cytotoxic T lymphocyte response and allogeneic tumor rejection. J Exp Med. 1991 Jul 1;174(1):193–201. doi: 10.1084/jem.174.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Kägi D., Aebischer T., Odermatt B., Held W., Podack E. R., Zinkernagel R. M., Hengartner H. Detection of perforin and granzyme A mRNA in infiltrating cells during infection of mice with lymphocytic choriomeningitis virus. Eur J Immunol. 1989 Jul;19(7):1253–1259. doi: 10.1002/eji.1830190716. [DOI] [PubMed] [Google Scholar]
- Nakata M., Smyth M. J., Norihisa Y., Kawasaki A., Shinkai Y., Okumura K., Yagita H. Constitutive expression of pore-forming protein in peripheral blood gamma/delta T cells: implication for their cytotoxic role in vivo. J Exp Med. 1990 Dec 1;172(6):1877–1880. doi: 10.1084/jem.172.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M., Pawankar R. Flow cytometric analysis of intraepithelial lymphocytes in the human nasal mucosa. Allergy. 1992 Jun;47(3):255–259. doi: 10.1111/j.1398-9995.1992.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Payer E., Strohal R., Kutil R., Elbe A., Stingl G. Demonstration of a CD3+ lymphocyte subset in the epidermis of athymic nude mice. Evidence for T cell receptor diversity. J Immunol. 1992 Jul 15;149(2):413–420. [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Kupfer A. T-cell effector functions: mechanisms for delivery of cytotoxicity and help. Annu Rev Cell Biol. 1991;7:479–504. doi: 10.1146/annurev.cb.07.110191.002403. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Yockey C. E., Brenner M. B., Balk S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993 Jul 1;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B. Characterization of V beta-bearing cells in athymic (nu/nu) mice suggests an extrathymic pathway for T cell differentiation. Eur J Immunol. 1990 Apr;20(4):919–925. doi: 10.1002/eji.1830200430. [DOI] [PubMed] [Google Scholar]
- Shivakumar S., Tsokos G. C., Datta S. K. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989 Jul 1;143(1):103–112. [PubMed] [Google Scholar]
- Sneller M. C., Straus S. E., Jaffe E. S., Jaffe J. S., Fleisher T. A., Stetler-Stevenson M., Strober W. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest. 1992 Aug;90(2):334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P. Autoimmune thyroiditis: predisposition and pathogenesis. Clin Endocrinol (Oxf) 1992 Apr;36(4):307–323. doi: 10.1111/j.1365-2265.1992.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Makgoba M. W., Borysiewicz L. K. Expression of an intercellular adhesion molecule, ICAM-1, by human thyroid cells. J Endocrinol. 1989 Jul;122(1):185–191. doi: 10.1677/joe.0.1220185. [DOI] [PubMed] [Google Scholar]
- Wirt D. P., Brooks E. G., Vaidya S., Klimpel G. R., Waldmann T. A., Goldblum R. M. Novel T-lymphocyte population in combined immunodeficiency with features of graft-versus-host disease. N Engl J Med. 1989 Aug 10;321(6):370–374. doi: 10.1056/NEJM198908103210606. [DOI] [PubMed] [Google Scholar]
- Young L. H., Joag S. V., Zheng L. M., Lee C. P., Lee Y. S., Young J. D. Perforin-mediated myocardial damage in acute myocarditis. Lancet. 1990 Oct 27;336(8722):1019–1021. doi: 10.1016/0140-6736(90)92486-2. [DOI] [PubMed] [Google Scholar]
- Young L. H., Peterson L. B., Wicker L. S., Persechini P. M., Young J. D. In vivo expression of perforin by CD8+ lymphocytes in autoimmune disease. Studies on spontaneous and adoptively transferred diabetes in nonobese diabetic mice. J Immunol. 1989 Dec 15;143(12):3994–3999. [PubMed] [Google Scholar]



