Abstract
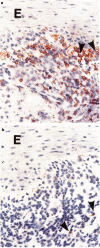
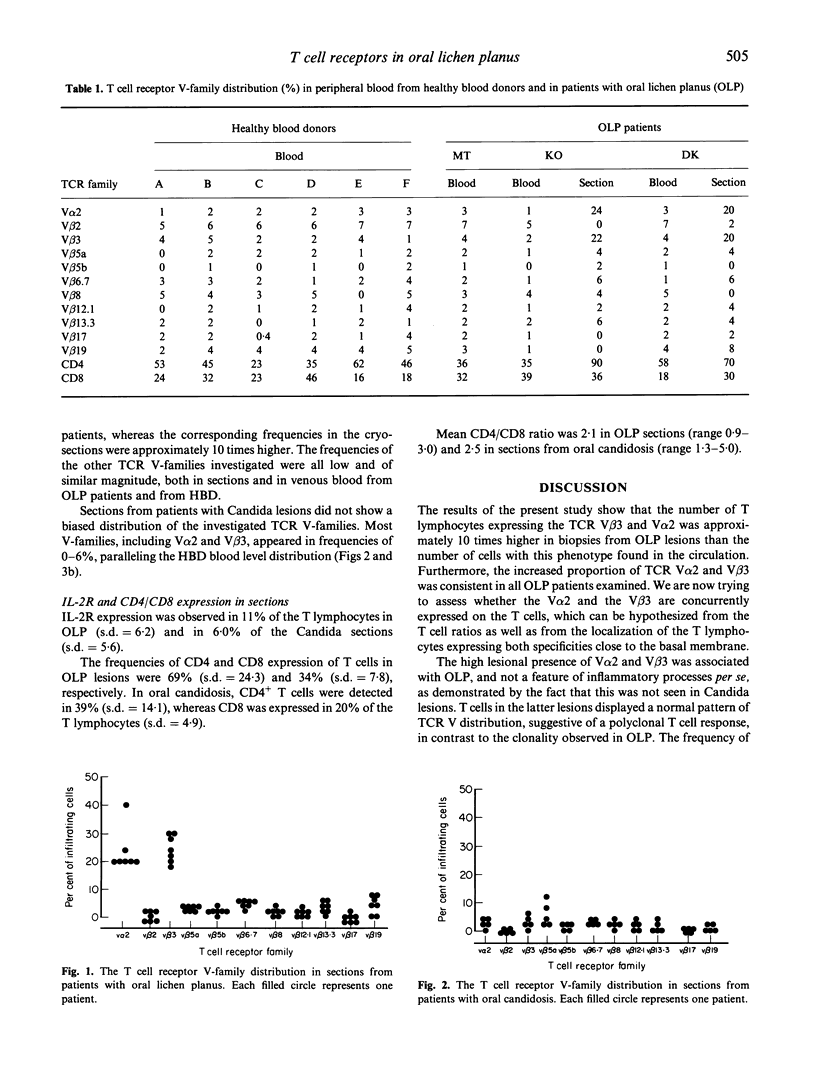
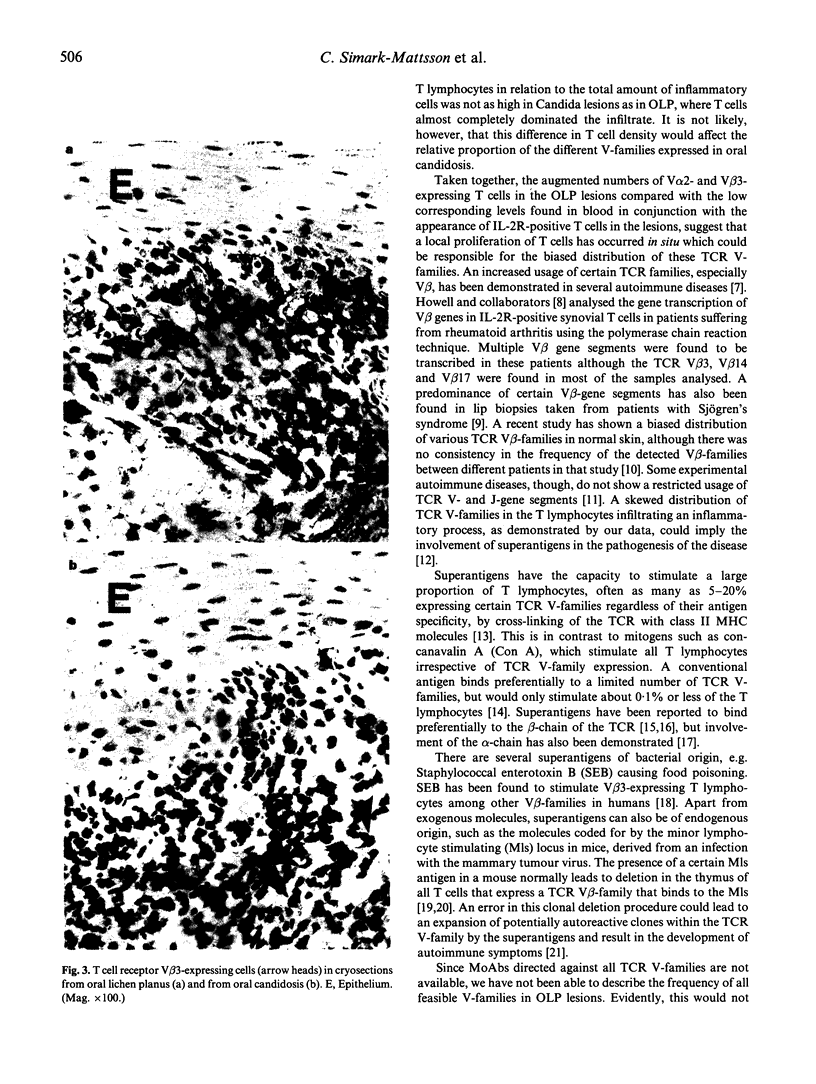
In order to analyse the clonality of T cells in the inflammatory infiltrate of oral lichen planus (OLP), mucosal biopsies were obtained from seven patients with manifest disease. The biopsies were stained with MoAbs directed against 11 different T cell receptor (TCR) V-gene families, anti-CD4, anti-CD8 and IL-2 receptor (IL-2R). For comparison, the frequencies of the different TCR V-families were determined in biopsies from five patients with oral candidosis as well as in peripheral blood from three patients with OLP and from six healthy blood donors (HBD). The occurrence of the investigated TCR V-families varied between 0% and 7% in venous blood obtained from both HBD and OLP patients. T lymphocytes expressing the TCR V beta 3 and V alpha 2 in OLP biopsies were, however, detected in frequencies ranging between 18% and 40% of the total fraction of lymphocytes, a consistent finding for all the OLP infiltrates studied. The other nine TCR V-families examined appeared in low frequencies both in biopsies and in peripheral blood. V alpha 2+ and V beta 3+ cells were often localized adjacent to the basal membrane. In contrast, T cells in Candida-induced lesions did not express a biased TCR distribution, and most V-families studied appeared in frequencies of 0-6%. Thus, T lymphocytes in OLP lesions express a substantially higher frequency of TCR V alpha 2 and V beta 3 than expected from the distribution in blood. The clonal expansion of T cells observed in OLP suggests that a superantigen is involved in the pathogenesis of the disease. Whether this superantigen is of exogenous or endogenous origin needs to be investigated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Held W., Waanders G. A., Shakhov A. N., Scarpellino L., Lees R. K., MacDonald H. R. Exogenous and endogenous mouse mammary tumor virus superantigens. Immunol Rev. 1993 Feb;131:5–25. doi: 10.1111/j.1600-065x.1993.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N., Young J. W., Nisanian A. J., Baggers J., Steinman R. M. Small amounts of superantigen, when presented on dendritic cells, are sufficient to initiate T cell responses. J Exp Med. 1993 Aug 1;178(2):633–642. doi: 10.1084/jem.178.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. A., Gadenne A. S., Simha S., Lerner E. A., Bigby M., Bleicher P. A. T-cell receptor V beta expression in normal human skin. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1267–1271. doi: 10.1073/pnas.90.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E., Acha-Orbea H. The V-region disease hypothesis: evidence from autoimmune encephalomyelitis. Immunol Today. 1989 May;10(5):164–169. doi: 10.1016/0167-5699(89)90174-6. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Hibberd M. L., Wong F. S., Nicholson L. B., Demaine A. G. Analysis of human T-cell receptor V beta gene usage following immunization to tetanus toxoid in vivo. Immunology. 1993 Jul;79(3):398–402. [PMC free article] [PubMed] [Google Scholar]
- Hirota J., Osaki T., Tatemoto Y. Immunohistochemical staining of infiltrates in oral lichen planus. Pathol Res Pract. 1990 Oct;186(5):625–632. doi: 10.1016/s0344-0338(11)80226-8. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserlian D., Vidal K., MacDonald H. R., Grosjean I. Mouse intestinal epithelial cells express the self superantigen Mls1a. Eur J Immunol. 1993 Oct;23(10):2717–2720. doi: 10.1002/eji.1830231053. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Klein M. H., Concannon P., Everett M., Kim L. D., Hunkapiller T., Hood L. Diversity and structure of human T-cell receptor alpha-chain variable region genes. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6884–6888. doi: 10.1073/pnas.84.19.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E., Concannon P., Hood L. Conserved organization of the human and murine T-cell receptor beta-gene families. Nature. 1988 Feb 11;331(6156):543–546. doi: 10.1038/331543a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Malmström M., Konttinen Y. T., Jungell P. Lymphocyte activation in oral lichen planus. Proc Finn Dent Soc. 1989;85(2):109–117. [PubMed] [Google Scholar]
- Marguerie C., Lunardi C., So A. PCR-based analysis of the TCR repertoire in human autoimmune diseases. Immunol Today. 1992 Sep;13(9):336–338. doi: 10.1016/0167-5699(92)90166-5. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Nakano N., Kikutani H., Nishimoto H., Kishimoto T. T cell receptor V gene usage of islet beta cell-reactive T cells is not restricted in non-obese diabetic mice. J Exp Med. 1991 May 1;173(5):1091–1097. doi: 10.1084/jem.173.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Bill J., Kubo R. T., Marrack P., Kappler J. W. Analysis of the interaction site for the self superantigen Mls-1a on T cell receptor V beta. J Exp Med. 1991 May 1;173(5):1183–1192. doi: 10.1084/jem.173.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida T., Yonaha F., Maeda T., Tanabe E., Koike T., Tomioka H., Yoshida S. T cell receptor repertoire of infiltrating T cells in lips of Sjögren's syndrome patients. J Clin Invest. 1992 Feb;89(2):681–685. doi: 10.1172/JCI115635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio M. S., Kanagawa O., Tomonari K., Hodes R. J. Influence of T cell receptor V alpha expression on Mlsa superantigen-specific T cell responses. J Exp Med. 1992 May 1;175(5):1405–1408. doi: 10.1084/jem.175.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L. J., Savage N. W., Ishii T., Seymour G. J. Immunopathogenesis of oral lichen planus. J Oral Pathol Med. 1990 Oct;19(9):389–396. doi: 10.1111/j.1600-0714.1990.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Zaller D. M., Osman G., Kanagawa O., Hood L. Prevention and treatment of murine experimental allergic encephalomyelitis with T cell receptor V beta-specific antibodies. J Exp Med. 1990 Jun 1;171(6):1943–1955. doi: 10.1084/jem.171.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]