Abstract
Individuals repeatedly exposed to HIV, but who remain uninfected, form a population enriched for persons likely to have either natural or acquired resistance to the virus. We have studied four such exposed uninfected cohorts, representing 60 individuals, for evidence of protective immunity. This population included participants exposed to HIV through anal or vaginal receptive intercourse on multiple occasions over many years. We observed CD8+-cell noncytotoxic inhibition of HIV replication in acutely infected CD4+ cells in the vast majority of individuals most recently exposed to the virus (within 1 year). The levels of this CD8+-cell response were sufficient to inhibit the in vitro infection of the exposed subjects’ peripheral blood mononuclear cells. We found no evidence of a significant role for CCR5 Δ32 mutation in this population, nor did CD4+ cell susceptibility to infection or HIV-specific cytotoxic T-lymphocytes correlate with resistance to infection in the individuals tested. Therefore, the observed strong noncytotoxic CD8+-cell anti-HIV responses may be an antiviral immune activity contributing to the apparent protection from infection in these exposed uninfected individuals.
The relative risk of infection by HIV correlates directly with the number of exposures to the virus (1, 2). However, within any exposed uninfected (EU) population, some individuals remain uninfected despite multiple unprotected encounters with HIV-infected partners. These highly exposed but uninfected individuals may represent a population that has some form of either acquired or natural protection from the virus (3, 4).
Studies focusing on EU populations have helped to identify both genetic and cellular factors associated with resistance to infection. Specifically, the CCR5 β-chemokine receptor has been identified as a coreceptor used by certain HIV-1 strains for entry into cells (5). Infection by these predominantly nonsyncytium-inducing strains can be blocked by the β-chemokines through competition for receptor binding sites. The HIV resistance of a small fraction of EU subjects has been attributed to an inherited genetic mutation, specifically a 32-bp deletion (Δ32) resulting in lack of expression of the CCR5 molecule (6, 7). Additionally, the presence of certain cellular immune responses, such as proliferation to HIV-derived peptides (8, 9) or HIV-specific cytotoxic T-lymphocyte (CTL) activity (10–13) have been reported in some of the EU subjects evaluated.
We and others have identified another form of cellular anti-HIV immune activity that is mediated by CD8+ cells and results in inhibition of virus replication in infected cells (14, 15). This response does not require HLA compatibility and does not involve cell killing. Importantly, a positive correlation has been found between this CD8+-cell immune response and a good clinical prognosis in infected individuals. The highest levels of this virus-suppressing activity are found in long-term survivors of HIV infection, although typically this response is absent in people with AIDS (16, 17).
Using several distinct EU cohorts, we have investigated the possible reasons for protection from HIV infection. We find that CD4+ cells from the EU participants studied are susceptible to in vitro infection with HIV-1 isolates, including those from their primary sexual partner. However, unlike CD8+ cells from unexposed control subjects, high levels of CD8+ cell noncytotoxic suppression of HIV replication are found in these EU individuals, particularly those with the most recent exposure to the virus. This anti-HIV activity may therefore contribute to the apparent protection from HIV infection observed in exposed but uninfected populations.
MATERIALS AND METHODS
Study Subjects.
Individuals exposed to HIV but who remain uninfected were collected from four distinct sources according to Table 1: Alabama Study Group (cohort A), Young Men’s Health Study (cohort B), Marin County Clinic (cohort C), and local referrals (cohort D). The designation of EU was based on multiple sexual or IV drug injection exposures to HIV over a period of 1–10 years. This EU population included both men and women with the major risk factors of unprotected sexual contact, occasionally accompanied by needle sharing, with HIV+ partners. The HIV+ participants evaluated include the seropositive partners from many of the EU subjects, as well as infected asymptomatic subjects followed in our laboratory. All enrolled EU individuals were negative for HIV by the following criteria: plasma ELISA for the HIV p24 antigen (Coulter), indirect immunofluorescent assay for serum anti-HIV antibodies (18), lymphocyte coculture analysis for virus recovery (19), and, in many cases, PCR analyses of peripheral blood mononuclear cells (PBMC). The presence of urine antibodies was assessed by using the Calypte urine ELISA (20). Additionally, five random individuals were evaluated for serum-neutralizing antibodies against their partner’s virus isolate by using standard procedures (21).
Table 1.
Demographic and risk factor profile of the EU population
| Cohort | Age, yr | Gender | Risk group | n | Last exposure, months | Number of exposures past 12 mo |
|---|---|---|---|---|---|---|
| A | 37.5 | Female | Unprotected vaginal intercourse | 16 | <1 to 72 | 0 to 60 |
| B | 29.4 | Male | Unprotected recept. anal intercourse | 16 | <1 to 18 | 0 to 24 |
| C | 38.3 | Mixed: 80% male 20% female | Unprotected sexual exposure | 10 | 1 to 20 | 0 to 20 |
| D | 46.4 | Mixed: 89% male 11% female | Unprotected sexual exposure† | 18 | <1 to 72 | 0 to 52 |
| Total | 60 |
Mean ages, gender breakdowns, HIV risk factors, as well as range of time since last exposure and number of exposures in the previous 12 months are shown for individuals in each cohort. n =number of individuals tested. See Materials and Methods for a further description of the cohorts.
Two individuals within this group also reported sharing needles with an infected partner.
When available, the HIV+ partners for these EU individuals were recruited for the isolation of their specific virus strain. Control blood samples were obtained from either the leukopac preparations of random blood donors (Irwin Memorial Blood Centers, San Francisco, CA) or local uninfected volunteers reporting no high-risk activities. This study received the approval of the Committee for Human Research, University of California, San Francisco, CA.
CCR5 Genotyping.
The cell pellet from each blood specimen was resuspended in specimen extraction buffer (10 mM KCl/10 mM Tris⋅HCl, pH 8.3/0.05% Tween 20/0.05% NP40/0.1 mg/ml proteinase K) to a final cell density of ≥2 × 106 cells per ml and incubated at 100°C for 30 min. The cell lysate was then diluted 10-fold with specimen extraction buffer without proteinase K and stored at −70°C. Each 100 μl PCR reaction consisted of cell lysate from ≈104 cells or 50 ng genomic DNA in 50 mM KCl/10 mM Tris⋅HCl, pH 8.3/2 mM MgCl2, 0.1 mM dATP, dGTP, dCTP, 0.2 mM dUTP, 0.4 μM each primer, SYC658 and SYC659, 5 units AmpliTaq, and 2 units AmpErase UNG (Perkin–Elmer). PCR conditions were 50°C for 2 min, 95°C for 1 min, followed by 30 cycles of 95°C for 20 sec and 60°C for 20 sec and finally holding at 72°C, by using the Perkin–Elmer Thermocycler 9600. A 177-bp and a 145-bp PCR product were amplified from the wild-type (+) and Δ32 alleles, respectively. CCR5 genotype was determined by analyzing 5 μl of each PCR reaction on a 3% NuSieve and 1% agarose gel (FMC).
PBMC Isolation and Subset Purification.
PBMC were prepared from whole blood by Ficoll-Hypaque (Sigma) gradient separation (19). The CD4+ and CD8+ cellular fractions were purified by using anti-CD4 or anti-CD8 antibody-coated immunomagnetic beads (Dynal, Great Neck, NY), as previously described (17).
Viruses.
The SF2 and SF33 strains of HIV-1, isolated in our laboratory and cultured solely in PBMC (22, 23), are resistant to the β-chemokines, MIP-1α, MIP-1β, and RANTES (24). Primary virus isolates were recovered from the PBMC of HIV+ partners of EU subjects (when available) and propagated in phytohemagglutinin-stimulated PBMC as described (19, 25). All of these primary isolates were phenotyped by using the MT2 assay (26) and were determined to be nonsyncytium-inducing. One-ml aliquots of these “mini” bulk cultures were frozen at −70°C and the tissue culture infectious dose required to infect 50% of PBMCs (TCID50) was determined for each virus isolate as described (27).
In Vitro Infection Assays.
PBMC or CD4+ cells isolated by using anti-CD4 immunomagnetic beads were infected in vitro by using previously described procedures (17). Briefly, the cells were pretreated with phytohemagglutinin (3 days, 3 μg/ml), washed, and 106 cells were resuspended in virus stocks at the TCID50 doses specified. Duplicate cultures of in vitro-infected cells were placed in 24-well plates and passed every 3–4 days, at which time the reverse transcriptase (RT) activity in the culture fluid was assessed (25).
Determination of HIV-Specific CTL Activity.
PBMC samples were tested for the presence of CTL activity after antigen-specific in vitro stimulation in both bulk culture and limiting dilution analysis (LDA) formats. The bulk culture in vitro stimulation strategy amplifies precursor CTL from >95% of HIV-1 infected individuals (28). Gag, Pol, and Nef antigens (vP1291 + vP1288 + vP1218) were used as stimulators for both the bulk and LDA assays. For the bulk stimulation, a responder:stimulator ratio of 4:1 was used. In the LDA, 20,000 stimulators per well were used to stimulate six serial 2-fold dilutions of responders starting at 100,000 cells per well and 24 replicates per dilution. IL-7 (330 units/ml) was used in both assays at day 0. Bulk in vitro stimulation and LDA cultures were tested on days 12 and 14, respectively, for the presence of CTL activity. The bulk CTL assays were performed by using 40, 20, 10, and 5:1 effector to target cell ratios for the undepleted samples and 40:1 for the CD4 and CD8 cell-depleted samples.
Acute Infection Assay for CD8+ Cell Noncytotoxic Anti-HIV Activity.
Acute suppression assays were conducted as previously described (17). In brief, CD4+ cells from uninfected control subjects were acutely infected with the specified HIV-1 isolate and were cultured both alone and in the presence of CD8+ cells at CD8+:CD4+ cell ratios of 0.5, 1, and 2:1 in growth medium containing 100 units/ml of human rIL-2 (generously provided by Glaxo Wellcome). These cell mixtures were placed in 96-well plates in a final volume of 200 μl per well and were incubated for 7–14 days at 37°C. Cell cultures were passed every 3–4 days by replacing half the culture supernatant with fresh medium. Collected supernatants were monitored for RT activity (25). Percent suppression was calculated by comparing the average value of RT activity in culture fluids from six control wells containing only CD4+ infected cells with the average RT activity in fluids from duplicate wells containing the same ratio of CD8+ and CD4+ cells together. Control CD8+ cells from an unexposed individual were included in each set of experiments. Standard deviations between replicate wells were routinely <10%.
Statistical Analyses.
Statistical evaluations of percent suppression between groups were conducted by using the nonparametric Mann–Whitney analysis. This value was calculated from experimental data by using the statview se+ graphics computer software program (Brain Power, Calabasas, CA). CCR5 genotype data were analyzed by using the Fisher’s Exact Test.
RESULTS
Demographic, Serologic, and Risk Factor Profiles of the EU Study Population.
The age, gender, and primary risk behavior profiles for the EU individuals involved in this study are presented in Table 1. Twenty-one women and 39 men were included among a total of 60 EU subjects evaluated. The study population was 63% Caucasian, 31% African-American, and 6% Hispanic. All participants reported multiple episodes of unprotected receptive intercourse with an HIV-infected partner, combined in two cases with IV-needle sharing. All EU subjects were HIV negative and demonstrated no evidence of HIV-specific serum antibodies. Individuals in cohorts A and D were also negative for urine antibodies against HIV-1. Finally, five randomly selected individuals evaluated possessed no serum-neutralizing activity against their primary partner’s virus isolate (data not shown).
CD4+ Cells from HIV-EU Individuals Are Susceptible to in Vitro Infection.
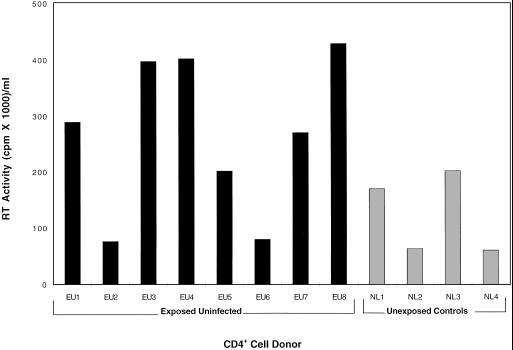
All 60 EU individuals were genotyped for the CCR5 Δ32 gene mutation, and these data were compared with similar typing conducted in a control unexposed population with shared ethnic backgrounds (29). Only two of the 60 EU subjects possessed a Δ32/Δ32 genotype, and no significant difference in the heterozygous genotype frequency was observed in this population (28%) as compared with 234 unexposed controls (23.4%; P = 0.064, data not shown). In additional studies designed to directly investigate the in vitro susceptibility of target cells from these individuals, purified CD4+ cells from eight randomly selected EU individuals and four unexposed control subjects who were not homozygous for CCR5 Δ32 were inoculated with 100 TCID50 of the SF2 virus strain. The CD4+ cells from these eight EU and four unexposed individuals were equally susceptible to HIV infection (Fig. 1). The CD4+ cells from five additional EU individuals for whom partner virus was available were also tested for susceptibility to these primary virus strains. In all of these experiments, random individuals from each of the four EU cohorts were included and no significant differences were detected when compared with CD4+ cells from control unexposed individuals (data not shown).
Figure 1.
Susceptibility of CD4+ cells from EU individuals to in vitro infection with HIV-1. The CD4+ cells from eight EU individuals (■) and four randomly selected unexposed control subjects (░⃞) were infected in vitro with HIV-1SF2 at 100 TCID50 per 106 cells. Evidence of virus production was measured by RT activity per ml of culture fluid collected at several time points post-inoculation. Data are shown for the day of peak virus replication (day 10).
CD8+ Cells from EU Individuals Suppress the Replication of HIV-1 in Vitro.
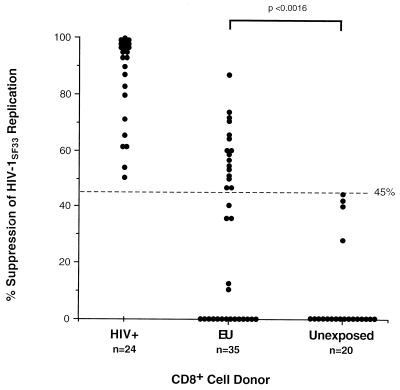
Strong noncytotoxic anti-HIV CD8+ cell activity resulting in the inhibition of viral replication has been found to correlate with protection from disease progression in infected individuals (16, 17). We investigated this same cellular immune response in our EU population by testing CD8+ cells from all 60 EU subjects for their ability to suppress one or more strains of HIV-1. Fig. 2 shows the actual percent suppression of SF33 virus replication mediated by CD8+ cells from 35 randomly selected EU individuals, 24 HIV+ participants, and 20 unexposed control subjects, at a CD8:CD4 cell ratio of 2:1. Nearly half of the 35 tested EU individuals, which included randomly selected subjects from each of the four cohorts, demonstrated levels of suppression greater than the maximum percentage observed in the control population (45%; see Fig. 2). By using this highly cytopathic, rapidly replicating and β-chemokine-insensitive virus strain, CD8+ cells from the EU individuals tested exhibited significantly greater levels of virus suppression than did CD8+ cells from unexposed controls (P < 0.0016; Fig. 2).
Figure 2.
CD8+ cell suppression of HIV-1SF33 virus replication in acutely infected CD4+ cells. The percent suppression of virus replication at a CD8+:CD4+ cell ratio of 2:1 is presented for 24 HIV+, 35 EU, and 20 unexposed (control) subjects. Statistical significance was determined by comparing the actual suppression values of all EU vs. unexposed individuals using a nonparametric Mann–Whitney analysis (P < 0.0016). A cutoff value of >45% suppression used to distinguish the EU population is shown by a dashed line.
Related studies were conducted by using less virulent virus strains, with a slower replication kinetics and a coreceptor usage more likely to match with strains of HIV to which EU individuals have been exposed. In these experiments, the CD8+ cells from 83% (15/18) and 72% (18/25) of the EU subjects evaluated demonstrated >90% inhibition of virus replication by using either a β-chemokine-sensitive primary virus isolate or the β-chemokine-resistant SF2 virus strain, respectively (data not shown). This cutoff value of 90% suppression is used to designate levels of activity significantly greater than those observed in any of the unexposed control individuals tested. There was a statistically significant difference between the levels of suppression seen in EU and unexposed groups (primary virus, P < 0.0003: SF2 virus, P < 0.0001). Importantly, the one CCR5 Δ32/Δ32 EU subject included in these assays was among those individuals demonstrating a strong CD8+ cell anti-HIV response (65% and 96% inhibition of SF33 and SF2, respectively).
Absence of HIV-1-Specific Cytotoxic T-Lymphocytes in EU Individuals.
PBMC from individuals participating in cohort A (see Table 1) were tested for the presence of HIV-specific CTL. By the bulk in vitro stimulation strategy, a method used to amplify precursor CTL from uninfected recipients of candidate AIDS vaccines (30, 31), 14/15 analyzed samples contained no evidence of CTL activity against any of the HIV antigens (Env, Gag, Pol, and Nef) tested. One sample showed a low anti-Gag (9%), anti-Pol (10%), and anti-Nef (10%) cytotoxicity. However, this response was found to be caused by the presence of CD4+ effector cells in the culture. The LDA technique also detected no significant HIV-specific CTL frequency in the 14 subjects evaluated with this procedure. At the same time, 4/5 (80%) and 6/14 (43%) of the individuals evaluated within this cohort demonstrated noncytotoxic CD8+ cell responses against the SF2 and SF33 virus isolates, respectively.
The Relative Risk of Infection Correlates with CD8+ Cell Noncytotoxic Anti-HIV Activity.
To investigate the relationship between the time since last exposure to HIV and this antiviral immune activity, the CD8+ cell noncytotoxic responses of individuals who had been sorted according to exposure history were compared with unexposed controls (Table 2). All EU participants were stratified by using the following criteria: “very high risk” if multiple exposures occurred in the last 6 months, “moderate risk” if the exposures occurred more than 6 months but less than 1 year ago, and “lower risk” if their exposures occurred over 1 year ago. In each of the cohorts studied, this CD8+ cell-mediated suppression of HIV was more likely to be present in individuals exposed within the last year (Table 2). This inhibition of virus replication was observed in 50–100% of very high-risk (VHR) subjects, 40–54% of medium-risk (MR) subjects, and 0–25% of lower-risk (LR) subjects, depending on the virus strain used. Likewise, none of the samples from unexposed controls demonstrated this level of antiviral immune activity.
Table 2.
Correlation of relative risk of infection with CD8+-cell noncytotoxic anti-HIV response
| Virus isolate tested | Fraction of
group demonstrating virus suppression
|
|||
|---|---|---|---|---|
| VHR | MR | LR | Unexp. | |
| HIV-1SF33 | 50% (n = 14) | 54% (n = 13) | 25% (n = 8) | 0% (n = 20) |
| HIV-1SF2 | 100% (n = 13) | 40% (n = 10) | 0% (n = 3) | 0% (n = 10) |
| HIV-1ALA33 | 100% (n = 12) | 50% (n = 6) | 0% (n = 2) | 0% (n = 8) |
Values shown are the fraction of individuals whose CD8+ cells demonstrate >45% inhibition of HIV-1SF33 (CXCR4-tropic, β-chemokine-resistant) or >90% inhibition of HIV-1SF2 (β-chemokine-resistant) and primary virus, HIV-1ALA33 (β-chemokine-sensitive). Inhibition of virus replication was assessed by RT levels in culture supernatants on days 3, 7, and 10 postinfection, with relative suppression determined on the day of peak virus activity. Subjects were stratified according to the time since their last unprotected exposure to an HIV-infected partner. VHR, very high risk (multiple exposures in the last 6 months). MR, moderate risk (multiple exposures >6 months ago, but within last year). LR, lower risk (multiple exposures >1 year ago). See Table 1 and Materials and Methods for further description of the cohorts. Unexp. = unexposed control subjects
The PBMC of EU Individuals with Strong Noncytotoxic CD8+ Cell Anti-HIV Responses Are Less Susceptible to in Vitro Infection.
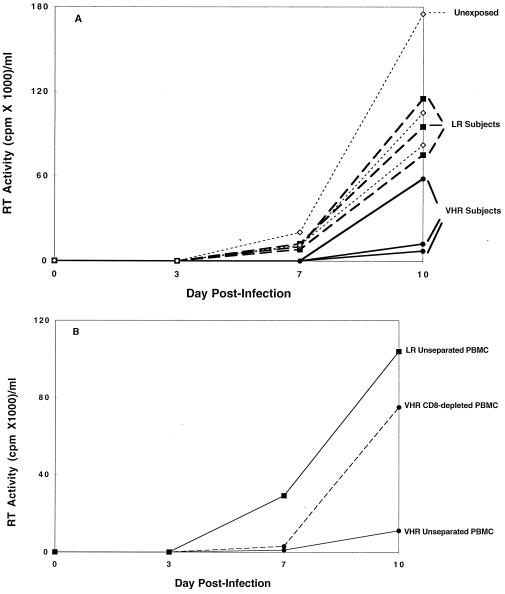
If CD8+ cell-mediated suppression of viral replication is a mechanism for resistance to infection, then this process should occur in vivo at the natural CD8+/CD4+ cell ratios found in the PBMC of EU individuals with this immune response. To test this hypothesis, in vitro SF2 virus infections were conducted by using the unseparated PBMC from randomly selected VHR or LR participants who did or did not possess this antiviral response, respectively. The SF2 strain was chosen because it is a well characterized slowly replicating isolate that is insensitive to the β-chemokines, therefore eliminating the need to account for any inhibitory effects mediated by these substances. The PBMC from the three VHR subjects with strong CD8+ cell noncytotoxic anti-HIV responses (>90%) showed the lowest levels of HIV replication (Fig. 3A). In contrast, PBMC from the three LR individuals who lacked this CD8+ cell response showed susceptibility to infection similar to that seen in PBMC from unexposed controls. Similar sets of experiments have been conducted on an additional five VHR, four LR, and two unexposed controls, all with similar results. Additional SF2 infections were carried out with CD8 cell-depleted PBMC vs. unseparated PBMC on these same five VHR, four LR, and two unexposed subjects. The results from these experiments confirm that inhibition of virus replication is abrogated with removal of the CD8+ cells (data not shown; for one example, see Fig. 3B).
Figure 3.
Acute infection of PBMC from EU and unexposed individuals. (A) Evidence of virus production was measured by RT activity in the culture fluids postinoculation with HIV-1SF2 (1, 10, or 100 TCID50/106 cells) on days 3, 7, and 10 postinfection. Data are presented for 100 TCID50 only. Virus production in the PBMC from three VHR individuals exhibiting strong CD8+ cell-noncytotoxic antiviral suppression (•) and three LR individuals lacking this response (■) are compared with the PBMC from three unexposed control subjects (◊). All assays were conducted in triplicate and the mean values of RT activity in culture fluids are presented. (B) Kinetics of virus replication (HIV-1SF2) in PBMC are shown for cells from one VHR individual exhibiting CD8+ cell anti-HIV activity (•) and one LR subject lacking this response (■). Either whole PBMC (solid lines) or CD8 cell-depleted PBMC (dashed line) were infected in vitro with 100 TCID50 of HIV-1SF2 and virus replication at days 3, 7, and 10 post-infection was assessed by RT activity in culture supernatants. All assays were conducted in triplicate and results are representative of three separate experiments. See Materials and Methods for a more detailed description of the procedures.
DISCUSSION
In determining important mechanisms for protection from HIV infection, individuals who remain seronegative despite multiple exposures to the virus represent an extremely valuable study population. The observation that in some people repeated exposure to HIV does not result in infection could be explained in several ways. First, low viral loads or the presence of replication-defective virus strains in the primary partner could result in a reduced rate of transmission. Second, resistance to infection in an EU individual may be caused by the absence or reduced susceptibility of target cells. Finally, protection from infection may be mediated by HIV-specific antibodies or cells that are capable of inhibiting infection and/or viral spread. Based on this latter mechanism, repeated exposures could potentially lead to enhancement of antiviral immunity, functioning much like a booster vaccination. In this regard, in vivo experiments in which macaques were exposed to subinfectious doses of SIV showed that these uninfected animals were protected from subsequent challenge with virus inocula (32).
Several potential correlates to protection in EU individuals were investigated in the present study. Particular attention was given to the relative risk of infection and the use of relevant virus isolates. Analyses of virus isolates showed that infectious virus of a nonsyncytium-inducing phenotype could be cultured from the PBMC of most of the HIV-infected sexual partners involved in this study (data not shown). Moreover, seminal fluid samples from the majority of the seropositive partners in cohort A were found to contain virus (D.L., unpublished results), supporting the conclusion that the uninfected partners of these individuals were likely to have been exposed to HIV.
The lack of a viral coreceptor on CD4+ cells can serve as a form of natural protection from HIV infection (6, 7). However, in the present study the CCR5 Δ32 deletion phenotype was observed in only two of the 60 EU individuals. We also found that the CD4+ cells of several randomly selected EU subjects were as susceptible to in vitro infection with HIV-1 isolates, including their partners’ isolate, as were those of their unexposed counterparts (Fig. 1). In particular, the use of virus from the primary partner to evaluate the HIV-susceptibility of CD4+ cells is a unique feature of this study.
In terms of antibody responses, no anti-HIV Igs were detected in the serum, urine, or vaginal secretions (D.L., unpublished work) of the EU subjects in cohort A. Likewise, none of five randomly tested individuals had serum-neutralizing antibodies against their partner’s virus isolate. These results conflict with those of a recent study reporting the existence of HIV-specific IgA in the urine and vaginal washes from a group of exposed but uninfected women (33). The reason for this discrepancy is unclear and could be the result of the limited numbers of individuals evaluated for these parameters.
In this report, the primary immunologic difference observed between the exposed and unexposed individuals was the strong CD8+ cell noncytotoxic antiviral activity in the EU group (Fig. 2 and Table 2). These data were obtained at CD8+:CD4+ cell ratios similar to those used to demonstrate antiviral responses in healthy HIV+ individuals (17) and by using β-chemokine-resistant as well as sensitive viral strains. The results indicate that this immune activity is both robust and not mediated by the β-chemokines. An important role for this antiviral immune response in preventing infection is also suggested by the observation that individuals demonstrating strong CD8+ cell anti-HIV immune responses had PBMC that were less sensitive to in vitro infection (Fig. 3A). Moreover, removal of the CD8+ cell population from PBMC abrogated this inhibitory effect (Fig. 3B). Finally, it is noteworthy that the CD8+ cells from one of the CCR5 Δ32/Δ32 VHR individuals showed strong virus-suppressing responses, although as expected the CD4+ cells from this person were refractory to in vitro replication of nonsyncytium-inducing virus strains (unpublished observation).
The inhibition of virus replication measured in the CD8+ cell assay described in this report is not the result of cellular cytotoxicity, an immune response that has been observed by others in EU populations (10–13). This conclusion is based on the following observations: (i) No HIV-specific CTLs were detected in cohort A; (ii) The CD8+ effector cell inputs used in the suppression assay were below that typically required to detect cytotoxicity (i.e., 40:1); and (iii) No effort was made to match target and effector cells for MHC antigens. One possible explanation for the apparent discrepancy in our CTL data as compared with others may be that cytotoxic responses were investigated in only a single cohort—those subjects with heterosexual risk factors. Additionally, cytotoxic activity against only three HIV-specific antigens was evaluated. We cannot rule out the possibility that these individuals harbor HIV-specific CTLs against regions that fall outside of these epitopes.
When EU individuals were stratified according to their relative risk of infection, the CD8+ cells from individuals reporting more recent incidents of exposure to virus demonstrated the greatest levels of virus inhibition (Table 2). This finding strongly suggests that exposure to HIV is needed for the induction of this immune activity and may indicate that the recall of this immune response is limited in duration. Subsequent exposures to HIV may act to enhance or expand this immune function.
The data presented in this report suggest that CD8+ cell antiviral activity is an important mechanism by which EU individuals remain uninfected after multiple exposures to the virus. Neither reduced CD4+ cell susceptibility to infection nor HIV-specific CTL activity was observed in the subset of subjects studied. The specific immune events surrounding an initial exposure (e.g., titer of virus or responding antigen-presenting cell type) may be important for eliciting protective immune responses in CD8+ cells. The discovery of this immunologic correlate to protection is encouraging for the future design of anti-HIV vaccines and could be used for the evaluation of vaccine preparations likely to induce protective immunity.
Acknowledgments
These studies were supported by grants from the California State University-wide AIDS Research Program (R96-SF1001) and the National Institutes of Health (RO1-AI29852). We thank Calypte Biomedical (Berkeley, CA) for their help in performing the urine HIV antibody assays and Bobby K. Joe for technical assistance. We are also grateful to Marcia Eisenberg at LabCorp America for providing the control samples for CCR5 genotyping studies.
ABBREVIATIONS
- EU
exposed uninfected
- CTL
cytotoxic T-lymphocyte
- PBMC
peripheral blood mononuclear cells
- LDA
limiting dilution analysis
- RT
reverse transcriptase
- VHR
very high risk
- LR
lower risk
- MR
moderate risk
- TCID50
tissue culture 50% infective dose
References
- 1.Ward J W, Bush T J, Perkins H A, Lieb L E, Allen J R, Goldfinger D, Samson S M, Pepkowitz S H, Fernando L P, Holland P V, et al. N Engl J Med. 1989;321:947–952. doi: 10.1056/NEJM198910053211406. [DOI] [PubMed] [Google Scholar]
- 2.Fowke K R, Nagelkerke N J D, Kimani J, Simonsen J N, Anzala A O, Bwayo J J, McDonald K S, Ngugi E N, Plummer F A. Lancet. 1996;348:1347–1351. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 3.Rowland-Jones S L, McMichael A. Curr Opin Immunol. 1995;7:448–455. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 4.Shearer G M, Clerici M. Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 6.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 7.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, van Devanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, et al. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 8.Ranki A, Mattinen S, Yarchoan R, Broder S, Ghrayeb J, Lahdevirta J, Krohn K. AIDS. 1989;3:63–69. doi: 10.1097/00002030-198902000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kelker H C, Seidlin M, Vogler M, Valentine F T. AIDS Res Hum Retroviruses. 1992;8:1355–1359. doi: 10.1089/aid.1992.8.1355. [DOI] [PubMed] [Google Scholar]
- 10.Cheynier R, Langlade-Demoyen P, Marescot M-R, Blanche S, Blondin G, Wain-Hobson S, Griscelli C, Vilmer E, Plata F. Eur J Immunol. 1992;22:2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 11.Langlade-Demoyen P, Ngo-Giang-Huong N, Ferchal F, Oksenhendler E. J Clin Invest. 1994;93:1293–1297. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 13.Pinto L A, Sullivan J, Berzofsky J A, Clerici M, Kessler H A, Landay A L, Shearer G M. J Clin Invest. 1995;96:867–876. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker C M, Moody D J, Stites D P, Levy J A. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 15.Brinchmann J E, Gaudernack G, Vartdal F. J Immunol. 1990;144:2961–2966. [PubMed] [Google Scholar]
- 16.Landay A L, Mackewicz C, Levy J A. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 17.Mackewicz C E, Ortega H W, Levy J A. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaminsky L S, McHugh T, Stites D, Volberding P, Henle G, Henle W, Levy J A. Proc Natl Acad Sci USA. 1985;82:5535–5539. doi: 10.1073/pnas.82.16.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro B A, Weiss C D, Wiviott L D, Levy J A. J Clin Microbiol. 1988;26:2371–2376. doi: 10.1128/jcm.26.11.2371-2376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urnovitz H B, Sturge J C, Gottfried T D. Nat Med. 1997;3:1258. doi: 10.1038/nm1197-1258. [DOI] [PubMed] [Google Scholar]
- 21.Homsy J, Meyer M, Levy J A. J Virol. 1990;64:1437–1440. doi: 10.1128/jvi.64.4.1437-1440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy J A, Hoffman A D, Kramer S M, Landis J A, Shimabukuro J M, Oshiro L S. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 23.Levy J A, Shimabukuro J M. J Infect Dis. 1985;152:734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- 24.Mackewicz C E, Barker E, Levy J A. Science. 1996;274:1393–1395. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman A D, Banapour B, Levy J A. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 26.Koot M, Vos A H V, Keet R P M, de Goede R E Y, Dercksen M W, Terpstra F G, Coutinho R A, Miedema F, Tersmette M. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 27.McDougal J S, Cort S P, Kennedy M S, Cabridilla C D, Feorino P M, Francis D P, Hicks D, Kalyanaraman V S, Martin L S. J Immunol Methods. 1985;76:171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari G, King K, Packard M V, Bartlett J A, Bolognesi D P, Weinhold K J. Clin Exp Immunol. 1995;43:283–294. doi: 10.1111/j.1365-2249.1995.tb08345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker E, Mackewicz C E, Reyes-Teran G, Sato A, Stranford S A, Fujimura S H, Christopherson C, Chang S Y, Levy J A. Blood. 1998;92:1–11. [PubMed] [Google Scholar]
- 30.Ferrari G, Humphrey W, McElrath M J, Excler J L, Duliege A M, Clements M L, Corey L C, Bolognesi D P, Weinhold K J. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElrath J M, Siliciano R F, Weinhold K J. AIDS Res Hum Retroviruses. 1997;13:211–216. doi: 10.1089/aid.1997.13.211. [DOI] [PubMed] [Google Scholar]
- 32.Clerici M, Clark E A, Polacino P, Axberg I, Kuller L, Casey N I, Morton W R, Shearer G M, Benveniste R E. AIDS. 1994;8:1391–1395. doi: 10.1097/00002030-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Mazzoli S, Trabattoni D, Caputo S L, Piconi S, Cle C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, et al. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]