Short abstract
A draft sequence of the compact genome of the sea squirt Ciona intestinalis illuminates how chordates originated and how vertebrate developmental innovations evolved.
Abstract
A draft sequence of the compact genome of the sea squirt Ciona intestinalis, a non-vertebrate chordate that diverged very early from other chordates, including vertebrates, illuminates how chordates originated and how vertebrate developmental innovations evolved.
"Mr. Kovalevsky has lately observed that the larvae of ascidians are related to the Vertebrata, in their manner of development, in the relative position of the nervous system, and in possessing a structure closely like the chorda dorsalis of vertebrate animals;... Thus, if we may rely on embryology, ever the safest guide in classification, it seems that we have at last gained a clew to the source whence the Vertebrata were derived."
Charles Darwin [1]
The organism
Early chordates were gentle, filter-feeding marine organisms, peaceful grazers of photosynthetic prokaryotes. Their descendants include rapacious vertebrates, with sense organs honed to detect prey, cunning nervous systems that out-wit hapless victims, and bony armor that protects them from counter-attack. What new genetic mechanisms led to the evolution of the innovative developmental programs that produced these novel vertebrate features? A recent article [2] announcing a draft genome sequence of the sea squirt Ciona intestinalis provides insights into not only the evolution of vertebrate novelties but also the entire phylum Chordata.
The adult C. intestinalis is a diaphanous sack that clings to a rock or to the piling of a pier, sucking in water through its mouth and filtering plankton through perforations - gill slits - in its pharynx. This simple adult 'sea squirt' disguised its true evolutionary heritage so well that for a long time it languished in classifications among the mollusks, or even the "Vermes". In 1866, Kovalevsky recognized that this 'gilt-rimmed vase' (see Figure 1a) and its relatives each developed from a beautiful larva that was obviously a chordate and a cousin of the vertebrates. The 'tadpole' larva has a notochord supporting a propulsive tail and a dorsal nervous system (Figure 1b). This microscopic larva settles from the plankton, glues its 'face' to a rock, and metamorphoses by resorbing its tail, restructuring its nervous system, and deploying a pair of siphons through which it will filter algae from the sea for the rest of its days.
Figure 1.
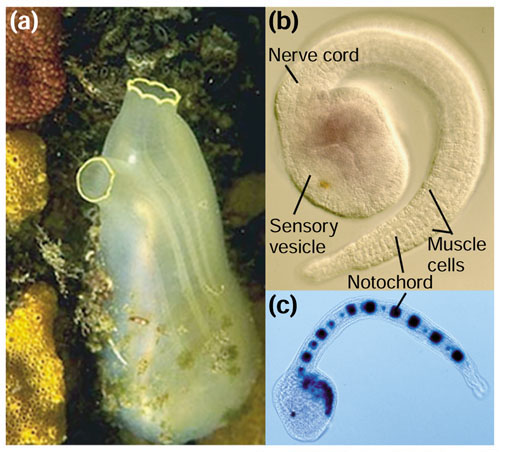
Ciona intestinalis.(a) The Ciona adult develops from a tadpole larva by a dramatic metamorphosis, during which it resorbs its tail, modifies its central nervous system, and transforms its digestive system into incurrent and excurrent siphons that filter plankton through perforations in the pharynx (gill slits). Image courtesy of Andrew Martinez. (b) An early tadpole hybridized with a probe for the alcohol dehydrogenase gene CiAdh3 to show the anterior endoderm [27]. Chordate features such as the notochord and the muscular tail are visible, and the positions of the sensory vesicle (brain) and dorsal nerve cord are indicated. (c) Electroporation is an efficient procedure by which to introduce DNA into Ciona for functional experiments, as is shown in this larva expressing a reporter construct made by Corbo et al. [4], a lacZ gene driven by the brachyury promoter in the notochord.
Since Kovalevsky's revelation, ascidians have generated considerable excitement in the discussion of vertebrate origins. Classical anatomists entertained the possibility that an ancient ascidian evolved a capacity to mature its gonads while remaining in the motile larval form, skipping a sessile adult stage, and that its descendents gave rise to the first vertebrate. Contemporary biologists acknowledge the evolutionary importance of ascidians differently - their genome and embryos offer a periscopic view on early chordate biology, but the ascidians themselves are not our ancestors.
Ascidians and their two sister classes, the larvaceans (or appendicularians) and thaliaceans, define the urochordates that, together with vertebrates and cephalochordates (such as amphioxus) make up the phylum Chordata (Figure 2). Urochordates split off earliest from other chordates, and the characteristics we share with them may therefore help to shape a portrait of the eochordate, the ancestor of modern chordates. Chordates, like echinoderms and hemichordates, are deuterostomes, a division of animals that are distinguished from other animals (protostomes) by the development of the embryonic blastopore into the anus rather than the mouth.
Figure 2.
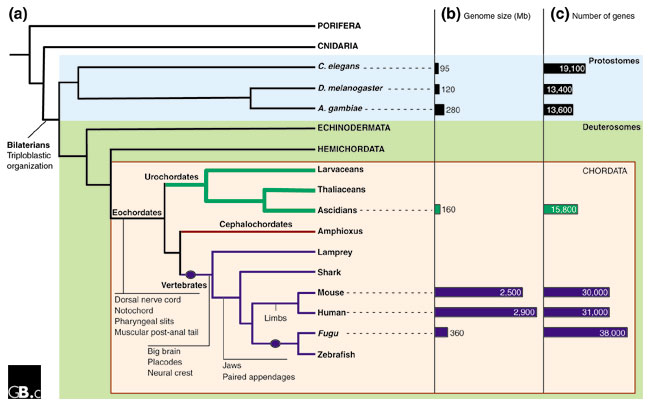
A phylogeny of chordate evolution. (a) A phylogenetic tree depicting the likely relationships between chordates and other animals, timing of genome amplifications (solid circles), and origin of novel characteristics. Phyla are in capitals; Porifera includes sponges; Cnidaria includes jellyfish and hydra; Echinodermata includes sea stars and sea urchins and Hemichordata includes marine acorn worms. (b) Genome size (Mb) and (c) estimated gene number of sequenced animal genomes are represented by bars.
Because of their phylogenetic position and their beautiful embryos, ascidians are popular among embryologists, and there has been impressive progress in the application of molecular tools to their study. These tools include loss-of-function studies using antisense morpholino oligonucleotides [3]; electroporation, which facilitates both the introduction of transgenes (Figure 1c) [4] and identification of cis-regulatory elements [5]; and the induction of mutations that block development in C. savignyi [6], a species whose genome is also being sequenced. The sequence of the C. intestinalis genome will greatly facilitate these functional analyses.
The sequence
A consortium of biologists and genomicists from sea coasts around the world (Australia, Canada, France, Italy, Japan, Scotland, and the USA), led by the US DOE Joint Genome Institute, used a whole-genome shotgun approach to determine the sequence of much of the 159 megabase (Mb) genome of a single C. intestinalis individual from California (Figure 2b). The size of the C. intestinalis genome is in the same range as genomes of protosomes; it is around 50 Mb larger than that of the nematode Caenorhabditis elegans and 100 Mb smaller than most insects [7]. The Ciona genome is only about 5% of the size of the human genome, and 43% of the dense genome of the pufferfish Fugu rubripes (365 Mb).
The Ciona sequence database [8] reports 116.7 Mb of unique sequence in 2,501 scaffolds longer than 3 kilobases (kb). Identification of genes and their annotation were facilitated by the Kyoto full-length cDNA project [9]. This has produced a database [10] that adds significant value to the sequence by providing expression data for thousands of sequences over several life-cycle stages. The team [2] identified 15,852 gene models in Ciona, a number comparable to other protostomes (there are 13,639 and 19,518 currently identified in D. melanogaster and C. elegans, respectively, according to the Ensembl project [11]) (Figure 2). On the other hand, this number is only about half the estimated total number of gene models in the human and mouse genomes (30,000, although at the time of this writing Ensembl predicted just 22,980 and 22,444 genes, respectively) [12]. F. rubripes appears to have an upper limit of about 38,000 genes [13] (or 35,180 according to Ensembl); this larger number is due to the genome duplication event that preceded the radiation of teleost fish [14].
The Ciona genome is tightly packed; it has 50% of the number of genes found in the human genome but only 5% of the quantity of DNA. A major factor influencing genome compaction is the abundance of interspersed repeats and transposable elements (TEs). In the mosquito Anopheles gambiae, for instance, TEs account for about 16% of the euchromatic genome and 60% of the heterochromatic genome; this contrasts with the compact genome of D. melanogaster with 2% and 8%, respectively [7]; and just 3% of the compact pufferfish genome is interspersed repeats, far below the 35-40% in mammals [13]. In Ciona, Dehal et al. [2] identify several high-copy-number repeat classes, which account for about 11% of the genome. Although this analysis does not describe any TEs, a previous systematic study identified several types in Ciona [15]. The Ciona genome draft now provides the raw data for an exhaustive analysis of TEs, which will illuminate their roles in evolutionary history of genome architecture.
The innovations
What does the Ciona sequence [2] tell us about the innovations of vertebrates, chordates, and deuterostomes? Almost 62% of Ciona genes (9,883) have a detectable protostome homolog, and these presumably constitute an ancient core of genes common to bilaterian animals. A few hundred Ciona genes, including phytochelatin synthase and hemocyanin, have stronger similarity to genes of protostomes than to any vertebrate gene. Either these are ancient bilaterian genes whose vertebrate orthologs have been lost or changed beyond recognition, or they were perhaps acquired by horizontal transfer from protostomes. Conversely, around 15% of Ciona genes (2,570) lack a clear protostome homolog yet have a vertebrate counterpart. These could have arisen in the deuterostome lineage after the split from protostomes, or alternatively they could be homologs of ancient bilaterian genes that have diverged beyond detection or been lost from modern protostomes. The genome sequence of an echinoderm, an outgroup to the chordates, will help determine if these genes are ancient within the deuterostomes [16].
Rather surprisingly, 21% of Ciona genes (3,399) have no clear homolog in the fly, worm, pufferfish, or human genomes (under high-stringency Smith-Waterman alignment along 60% of the target protein). Although this group might include poorly modeled genes or genes that have been broken by the ends of contigs, some may also have evolved so rapidly that they have lost significant resemblance to their orthologs, or they may be genes specific to urochordates or to ascidians. Resolution of this last ambiguity would require the sequencing of a distant urochordate genome, such as that of a larvacean [17].
What can the Ciona sequence tell us about vertebrate innovations? It can expedite the hunt for genes that are important in the development of the embryonic tissues that are widely believed to have initiated the evolution of the vertebrates [18]. These include neural crest and ectodermal placodes, which contribute to the paired sensory organs and head skeleton that are so apparent in vertebrates but are seemingly lacking from other chordates. If these tissues evolved after vertebrate origins, what functions do crest and placode genes have in an ascidian? Might such genes reveal the evolutionary precursors of these tissues? Many genes central to these questions have already been investigated in ascidians by targeted sequencing [19], but the Ciona sequencing project has detected more peripheral players. For instance, three Ciona genes (ci0100131069, ci0100130876 and ci0100135383) appear to be homologous to vertebrate genes encoding the olfactomedm family; one vertebrate member of this family, Noelin-1, encodes a protein that makes the neural tube competent to generate neural crest cells [20]. Two ascidian genes (ci0100149361 and ci0100140298) are homologous to the vertebrate HAND1 and HAND2 genes, which are also Important In crest development [21]. And Ciona has two orthologs (ci0100136347 and ci0100130219) of the vertebrate Prox1 gene, a marker of lens, otic, olfactory, and ganglia placodes [22]. The discovery of genes with only weak homology to olfactory receptors, however, undermines the prospects of discovering an olfactory placode precursor. Nonetheless, developmental biologists will be kept busy for some time studying the expression and function of these newly revealed genes.
Adaptive immunity involving lymphocytes appears to have arisen within the vertebrates; Dehal et al. [2] could not find Ciona homologs of genes involved in this system, including immunoglobulins, T-cell receptors, or major histocompatability complex (MHC) genes. Furthermore, they found that, although Ciona has orthologs for each of the 14 proteasome genes, it lacks immunoproteasome-specific genes, suggesting that Ciona has no specific system for presenting antigens. Nevertheless, ascidians have a potent innate immune system [23], including possible complement genes and several lectins.
Genome amplifications
Gene counts show that mice and humans have about twice as many genes as ascidians. When did genome-amplification events happen with respect to the origin of vertebrate innovations? Did amplification occur by whole-genome duplication or by the independent amplification of many small regions of the genome? And if it occurred by genome duplication, was there one round of duplication or more? A sequenced non-vertebrate chordate genome fully aligned along complete chromosomes would provide data with which to critically examine these issues. Although about 85% of the available Ciona sequence is in 905 scaffolds longer than 20 kb, these are not yet aligned along chromosomes. Substantial value would be added to the sequence if a meiotic or radiation-hybrid mapping panel were constructed, as a way to order markers from each scaffold. Then, long-range comparative synteny analysis could be brought to bear on the issue of the mechanism of vertebrate genome amplification.
Unfortunately, few ancient branches survive on the chordate tree (Figure 2). But the extant lineages diverged at key nodes for resolving important questions about chordate evolution. The sequence of a larvacean, a new model for molecular development [24] with a genome half the size of the ascidian [25], would allow generalizations about ancestral urochordates. Cephalochordates are the sister group of vertebrates, and the genome sequence of an amphioxus would, in conjunction with urochordate sequences, suggest the content of the genome before the genome amplification events that occurred with, and probably facilitated, the origin of vertebrate developmental novelties [26]. Finally, we need genomic evidence from the earliest diverging vertebrate whose embryos can be studied, namely a lamprey. Sifting through these genomes to design functional experiments on developmental regulatory mechanisms will help us learn how meek, unassuming grazers evolved into vicious predators like you and me.
Acknowledgments
Acknowledgements
We thank an NSF IGERT program grant DGE 9972830 in Evolution of Development and Genomics (S.B.), a grant EX2002-0059 from the Ministry of Education, Culture and Sports from the Spanish Government (C.C.), and NIH grant R01RR10715 (J.H.P.) for support.
References
- Darwin C. The Descent of Man, and Selection in Relation to Sex. New York: Hurst. 1874.
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomoso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Satou Y, Imai KS, Satoh N. Action of morpholinos in Ciona embryos. Genesis. 2001;30:103–106. doi: 10.1002/gene.1040. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Levine M, Zeller RW. Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development. 1997;124:589–602. doi: 10.1242/dev.124.3.589. [DOI] [PubMed] [Google Scholar]
- Harafuji N, Keys DN, Levine M. Genome-wide identification of tissue-specific enhancers in the Ciona tadpole. Proc Natl Acad Sci USA. 2002;99:6802–6805. doi: 10.1073/pnas.052024999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody R, Davis SW, Cubas F, Smith WC. Isolation of developmental mutants of the ascidian Ciona savignyi. Mol Gen Genet. 1999;262:199–206. doi: 10.1007/s004380051075. [DOI] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- JGI: Ciona intestinalis v1.0 http://genome.jgi-psf.org/ciona4/ciona4.home.html
- Satou Y, Takatori N, Fujiwara S, Nishikata T, Saiga H, Kusakabe T, Shin-i T, Kohara Y, Satoh N. Ciona intestinalis cDNA projects: expressed sequence tag analyses and gene expression profiles during embryogenesis. Gene. 2002;287:83–96. doi: 10.1016/s0378-1119(01)00826-5. [DOI] [PubMed] [Google Scholar]
- Ghost Database: a Ciona intestinalis cDNA resource http://ghost.zool.kyoto-u.ac.jp/indexr1.html
- Ensembl Genome Browser http://www.ensembl.org/
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Aparicio S, Chapman J, Stupk E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, et al. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297:1301–1310. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- Amores A, Force A, Yan Y-L, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang Y-L, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Simmen MW, Bird A. Sequence analysis of transposable elements in the sea squirt, Ciona intestinalis. Mol Biol Evol. 2000;17:1685–1694. doi: 10.1093/oxfordjournals.molbev.a026267. [DOI] [PubMed] [Google Scholar]
- Cameron RA, Mahairas G, Rast JP, Martinez P, Biondi TR, Swartzell S, Wallace JC, Poustka AJ, Livingston BT, Wray GA, et al. A sea urchin genome project: sequence scan, virtual map, and additional resources. Proc Natl Acad Sci USA. 2000;97:9514–9518. doi: 10.1073/pnas.160261897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Satoh N. Details of the evolutionary history from invertebrates to vertebrates, as deduced from the sequences of 18S rDNA. Proc Natl Acad Sci USA. 1994;91:1801–1804. doi: 10.1073/pnas.91.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–274. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Holland ND. Evolution of neural crest and placodes: amphioxus as a model for the ancestral vertebrate? J Anat. 2001;199:85–98. doi: 10.1046/j.1469-7580.2001.19910085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Molecular analysis of neural crest formation. J Physiol Paris. 2002;96:3–8. doi: 10.1016/s0928-4257(01)00074-2. [DOI] [PubMed] [Google Scholar]
- Howard M, Foster DN, Cserjesi P. Expression of HAND gene products may be sufficient for the differentiation of avian neural crest-derived cells into catecholaminergic neurons in culture. Dev Biol. 1999;215:62–77. doi: 10.1006/dbio.1999.9450. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Niedenfuhr M, Papoutsi M, Christ B, Nicolaides KH, von Kaisenberg CS, Tomarev SI, Wilting J. Prox1 is a marker of ectodermal placodes, endodermal compartments, lymphatic endothelium and lymphangioblasts. Anat Embryol (Berl) 2001;204:399–406. doi: 10.1007/s00429-001-0214-9. [DOI] [PubMed] [Google Scholar]
- Davidson B, Swalla BJ. A molecular analysis of ascidian metamorphosis reveals activation of an innate immune response. Development. 2002;129:4739–4751. doi: 10.1242/dev.129.20.4739. [DOI] [PubMed] [Google Scholar]
- Bassham S, Postlethwait J. Brachyury (T) expression in embryos of a larvacean urochordate, Oikopleura dioica, and the ancestral role of brachyury. Dev Biol. 2000;220:322–333. doi: 10.1006/dbio.2000.9647. [DOI] [PubMed] [Google Scholar]
- Seo H-C, Kube M, Edvardsen RB, Jensen MF, Beck A, Spriet E, Gorsky G, Thompson EM, Lehrach H, Reinhardt R, et al. Miniature genome in the marine chordate Oikopleura dioica. Science. 2001;294:2506. doi: 10.1126/science.294.5551.2506. [DOI] [PubMed] [Google Scholar]
- Holland PWH, Garcia-Fernàndez J, Williams NA, Sidow A. Gene duplications and the origins of vertebrate development. Dev. 1994;suppl:125–133. [PubMed] [Google Scholar]
- Cañestro C, Godoy L, Gonzàlez-Duarte R, Albalat R. Comparative expression analysis of Adh3 during arthropod, urochordate, cephalochordate and vertebrate development challenges its predicted housekeeping role. Evol Dev. [DOI] [PubMed]


