Abstract
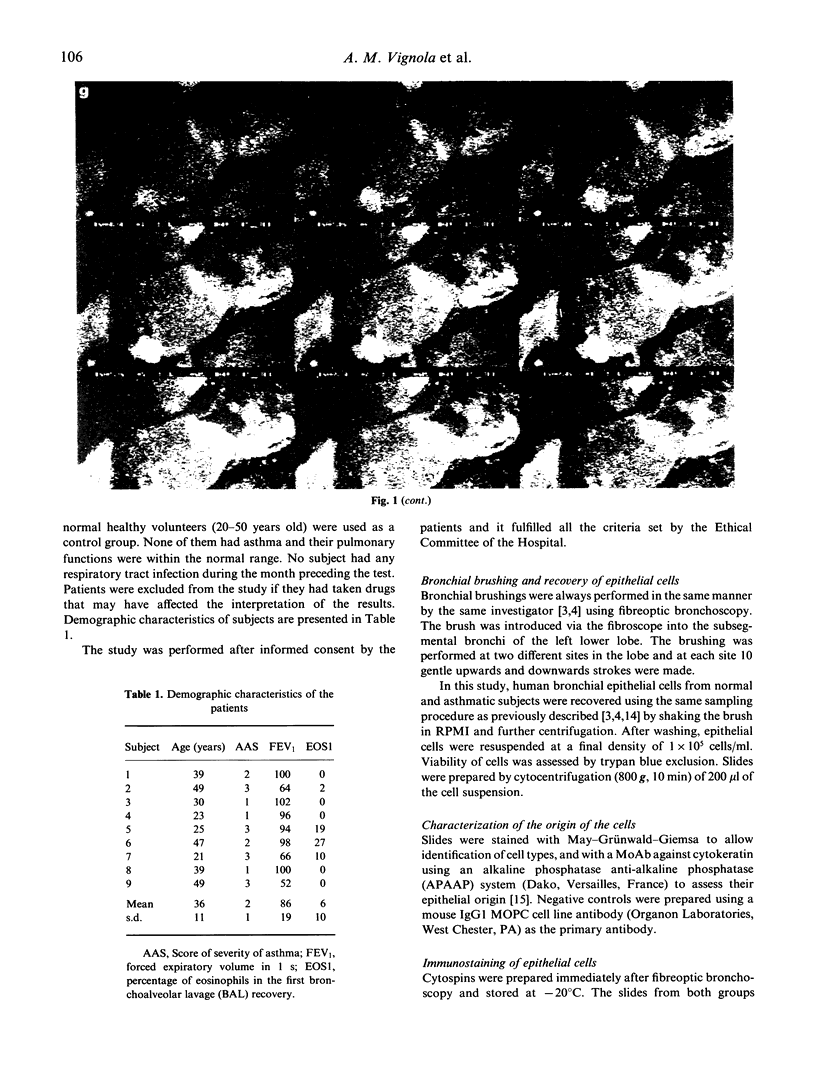
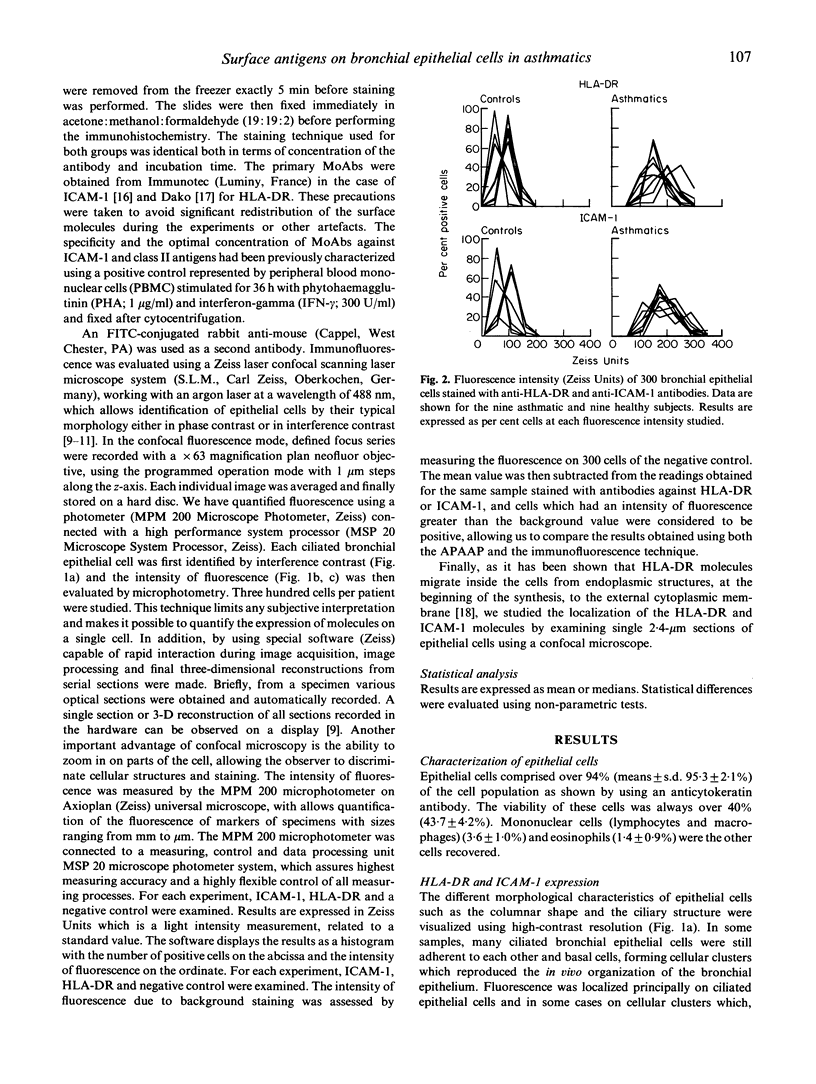
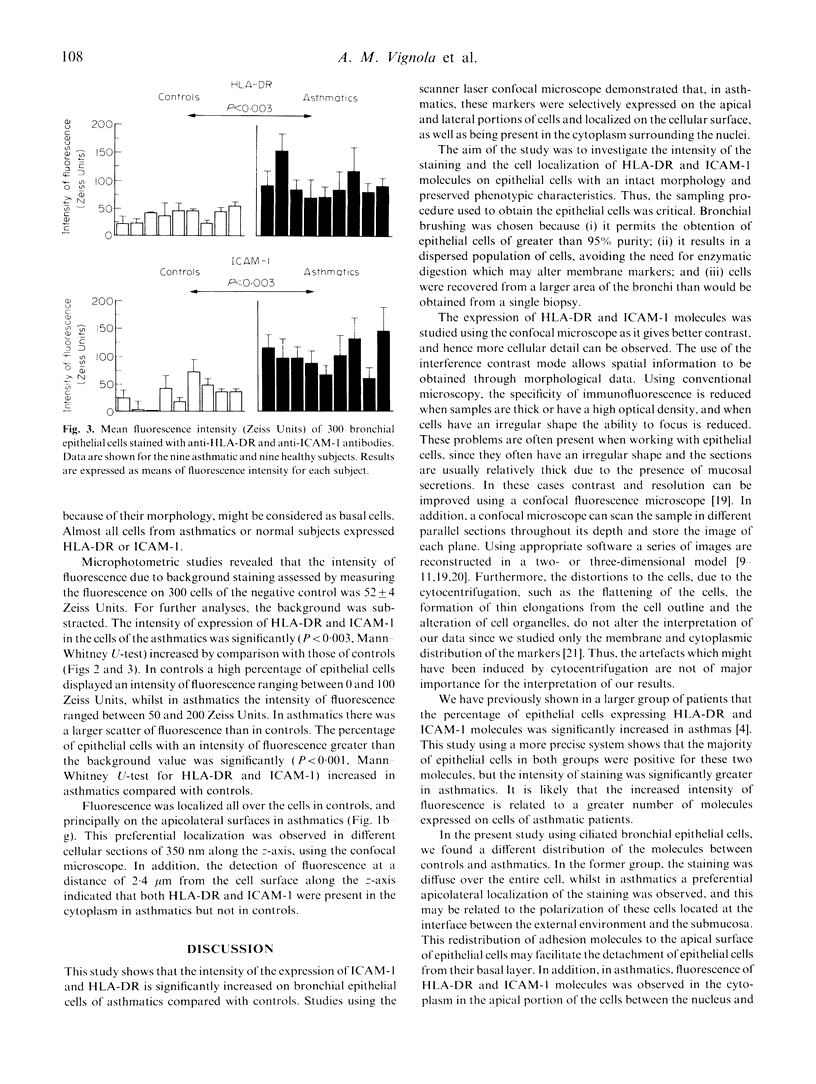
An increased expression of HLA-DR and ICAM-1 molecules on bronchial epithelial cells has been observed in asthmatic patients. The aim of this study was to evaluate the localization and to quantify the spontaneous expression of HLA-DR and ICAM-1 on bronchial epithelial cells recovered by bronchial brushing of nine asthmatics and nine controls. Epithelial cells constituted over 95% of cells recovered as shown using an anti-cytokeratin MoAb. Expression of HLA-DR and ICAM-1 was studied using indirect immunofluorescence and confocal microscopy. The intensity of fluorescence of epithelial cells expressing HLA-DR and ICAM-1 was significantly (P < 0.003) increased in asthmatics. In asthmatics, but not in controls, the expression of both molecules was localized in the cytoplasm on the apicolateral portions of the cells. This study shows an up-regulation in the expression of HLA-DR and ICAM-1 molecules by bronchial epithelial cells from asthmatics and a localization of these molecules within the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991 Mar;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- Bousquet J., Chanez P., Lacoste J. Y., Barnéon G., Ghavanian N., Enander I., Venge P., Ahlstedt S., Simony-Lafontaine J., Godard P. Eosinophilic inflammation in asthma. N Engl J Med. 1990 Oct 11;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Brakenhoff G. J., van Spronsen E. A., van der Voort H. T., Nanninga N. Three-dimensional confocal fluorescence microscopy. Methods Cell Biol. 1989;30:379–398. doi: 10.1016/s0091-679x(08)60987-5. [DOI] [PubMed] [Google Scholar]
- Campbell A. M., Chanez P., Vignola A. M., Bousquet J., Couret I., Michel F. B., Godard P. Functional characteristics of bronchial epithelium obtained by brushing from asthmatic and normal subjects. Am Rev Respir Dis. 1993 Mar;147(3):529–534. doi: 10.1164/ajrccm/147.3.529. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Crandall E. D., Kim K. J. Protein traffic across lung epithelia. Am J Respir Cell Mol Biol. 1989 Oct;1(4):255–255. doi: 10.1165/ajrcmb/1.4.255. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Fournier M., Lebargy F., Le Roy Ladurie F., Lenormand E., Pariente R. Intraepithelial T-lymphocyte subsets in the airways of normal subjects and of patients with chronic bronchitis. Am Rev Respir Dis. 1989 Sep;140(3):737–742. doi: 10.1164/ajrccm/140.3.737. [DOI] [PubMed] [Google Scholar]
- Kalb T. H., Chuang M. T., Marom Z., Mayer L. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol. 1991 Apr;4(4):320–329. doi: 10.1165/ajrcmb/4.4.320. [DOI] [PubMed] [Google Scholar]
- Montefort S., Holgate S. T. Adhesion molecules and their role in inflammation. Respir Med. 1991 Mar;85(2):91–99. doi: 10.1016/s0954-6111(06)80284-2. [DOI] [PubMed] [Google Scholar]
- Noah T. L., Paradiso A. M., Madden M. C., McKinnon K. P., Devlin R. B. The response of a human bronchial epithelial cell line to histamine: intracellular calcium changes and extracellular release of inflammatory mediators. Am J Respir Cell Mol Biol. 1991 Nov;5(5):484–492. doi: 10.1165/ajrcmb/5.5.484. [DOI] [PubMed] [Google Scholar]
- Odum N., Martin P. J., Schieven G. L., Hansen J. A., Ledbetter J. A. Signal transduction by HLA class II antigens expressed on activated T cells. Eur J Immunol. 1991 Jan;21(1):123–129. doi: 10.1002/eji.1830210119. [DOI] [PubMed] [Google Scholar]
- Peters P. J., Neefjes J. J., Oorschot V., Ploegh H. L., Geuze H. J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991 Feb 21;349(6311):669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Peão M. N., Aguas A. P., de Sá C. M., Grande N. R. Structural artifacts and advantages of cytocentrifugation of cells as viewed by scanning electron microscopy. Scanning Microsc. 1992 Mar;6(1):281–285. [PubMed] [Google Scholar]
- Poston R. N., Chanez P., Lacoste J. Y., Litchfield T., Lee T. H., Bousquet J. Immunohistochemical characterization of the cellular infiltration in asthmatic bronchi. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):918–921. doi: 10.1164/ajrccm/145.4_Pt_1.918. [DOI] [PubMed] [Google Scholar]
- Rossi G. A., Sacco O., Balbi B., Oddera S., Mattioni T., Corte G., Ravazzoni C., Allegra L. Human ciliated bronchial epithelial cells: expression of the HLA-DR antigens and of the HLA-DR alpha gene, modulation of the HLA-DR antigens by gamma-interferon and antigen-presenting function in the mixed leukocyte reaction. Am J Respir Cell Mol Biol. 1990 Nov;3(5):431–439. doi: 10.1165/ajrcmb/3.5.431. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Lennert K., Feller A. C., Mason D. Y. Immunohistological analysis of human lymphoma: correlation of histological and immunological categories. Adv Cancer Res. 1984;42:67–147. doi: 10.1016/s0065-230x(08)60456-x. [DOI] [PubMed] [Google Scholar]
- Vignola A. M., Campbell A. M., Chanez P., Bousquet J., Paul-Lacoste P., Michel F. B., Godard P. HLA-DR and ICAM-1 expression on bronchial epithelial cells in asthma and chronic bronchitis. Am Rev Respir Dis. 1993 Sep;148(3):689–694. doi: 10.1164/ajrccm/148.3.689. [DOI] [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Stelzer E. H., Wijnaendts-van-Resandt R. W., Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol. 1987 Oct;105(4):1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]





