Abstract
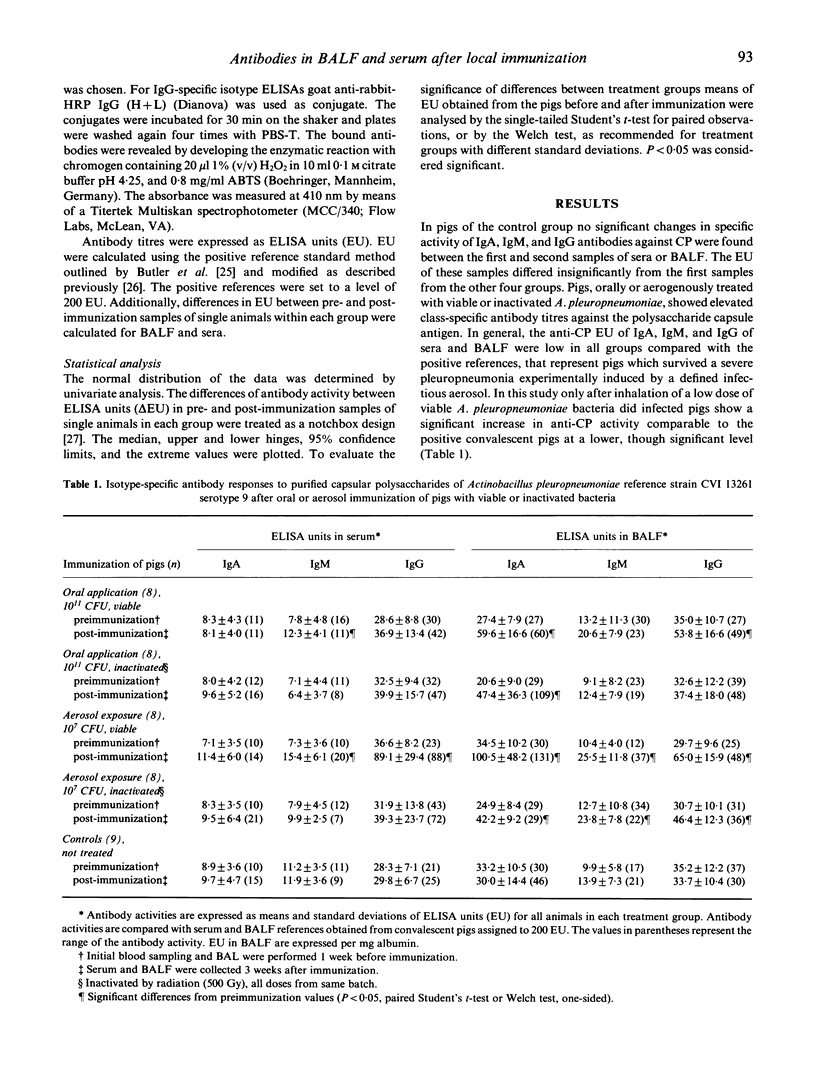
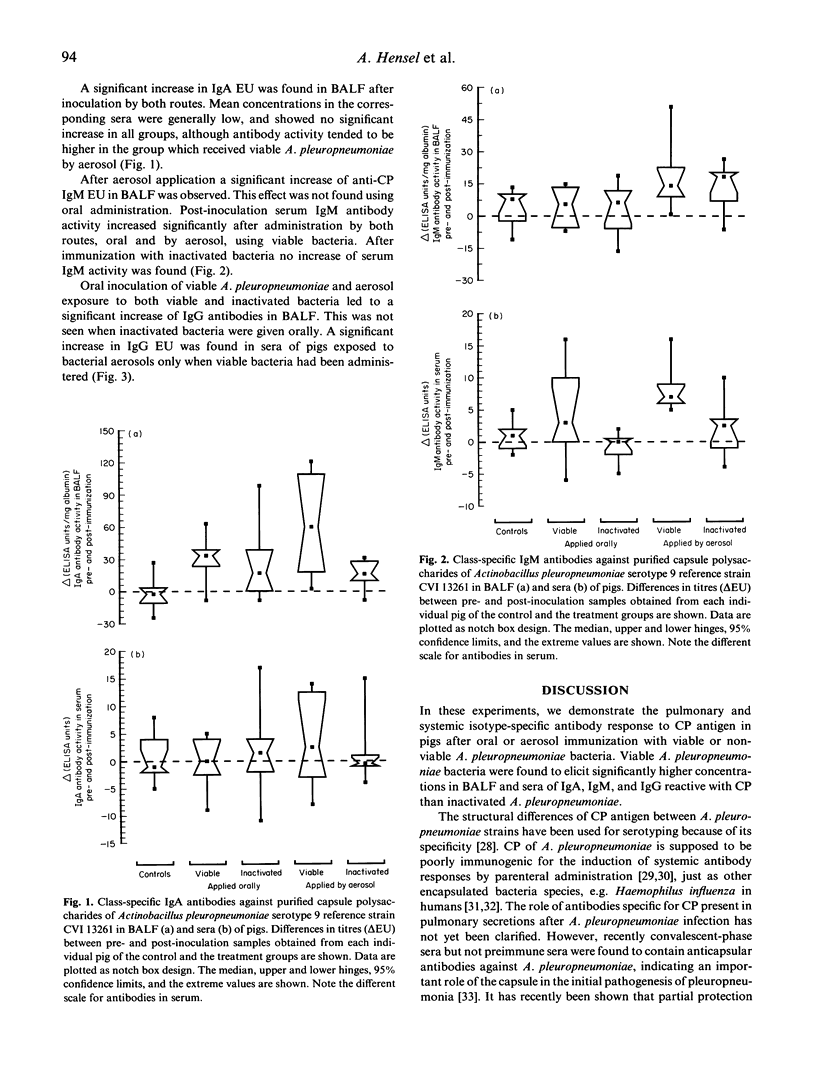
To investigate the antibody response after local application of lung-pathogenic bacteria, pigs were immunized with viable or inactivated Actinobacillus pleuropneumoniae by the oral and aerogenous route. After 3 weeks class-specific immunoglobulins against purified A. pleuropneumoniae capsular polysaccharides (CP) were determined in serum and BALF by ELISA. A significant increase of IgA antibodies was found in BALF but not in sera of all immunized pigs. Oral immunization with viable A. pleuropneumoniae and aerosol immunization with either viable or inactivated bacteria resulted in a significant increase of IgG antibodies to the CP antigen in BALF, whereas only aerosol exposure to viable bacteria resulted in a significant increase in IgG antibodies in serum. A significant increase in anti-CP IgM in BALF was observed after aerosol exposure but not after oral immunization. IgM antibodies towards CP increased significantly by both routes of immunization with viable bacteria. The anti-CP activity of all three isotypes in sera and BALF was low in all groups compared with the positive controls, although inoculation of viable A. pleuropneumoniae led to higher levels of antibody concentration than inactivated bacteria. Our results indicate a traffic of primed lymphocytes from the gut into the bronchoalveolar airways and further support the hypothesis that polysaccharide-specific B cells may functionally mature at the mucosal surfaces.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E., Robinson A. Oral immunization with bacterial polysaccharide and adjuvant enhances antigen-specific pulmonary secretory antibody response and resistance to pneumonia. Vaccine. 1991 Oct;9(10):757–764. doi: 10.1016/0264-410x(91)90293-f. [DOI] [PubMed] [Google Scholar]
- Bergmann K. C., Waldman R. H. Oral immunization with influenza virus: experimental and clinical studies. Curr Top Microbiol Immunol. 1989;146:83–89. doi: 10.1007/978-3-642-74529-4_9. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Gray R. H., Evans M. J., Muggenburg B. A. Identification of plasma cells in lung alveoli and interstitial tissues after localized lung immunization. J Leukoc Biol. 1987 Jan;41(1):1–7. doi: 10.1002/jlb.41.1.1. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Weissman D. N., Muggenburg B. A. Long-term maintenance of localized antibody responses in the lung. Immunology. 1991 Oct;74(2):215–222. [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Bossé J. T., Johnson R. P., Nemec M., Rosendal S. Protective local and systemic antibody responses of swine exposed to an aerosol of Actinobacillus pleuropneumoniae serotype 1. Infect Immun. 1992 Feb;60(2):479–484. doi: 10.1128/iai.60.2.479-484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. E., Feldbush T. L., McGivern P. L., Stewart N. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry. 1978 Feb;15(2):131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- Byrd W., Kadis S. Preparation, characterization, and immunogenicity of conjugate vaccines directed against Actinobacillus pleuropneumoniae virulence determinants. Infect Immun. 1992 Aug;60(8):3042–3051. doi: 10.1128/iai.60.8.3042-3051.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. S., Burlington D. B., Quinnan G. V., Jr Active synthesis of hemagglutinin-specific immunoglobulin A by lung cells of mice that were immunized intragastrically with inactivated influenza virus vaccine. J Virol. 1987 Jul;61(7):2150–2154. doi: 10.1128/jvi.61.7.2150-2154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R., Cripps A., Murree-Allen K., Yeung S., Engel M. Oral immunisation with killed Haemophilus influenzae for protection against acute bronchitis in chronic obstructive lung disease. Lancet. 1985 Dec 21;2(8469-70):1395–1397. doi: 10.1016/s0140-6736(85)92559-0. [DOI] [PubMed] [Google Scholar]
- Daniele R. P. Immunoglobulin secretion in the airways. Annu Rev Physiol. 1990;52:177–195. doi: 10.1146/annurev.ph.52.030190.001141. [DOI] [PubMed] [Google Scholar]
- Delventhal S., Hensel A., Petzoldt K., Pabst R. Cellular changes in the bronchoalveolar lavage (BAL) of pigs, following immunization by the enteral or respiratory route. Clin Exp Immunol. 1992 Nov;90(2):223–227. doi: 10.1111/j.1365-2249.1992.tb07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delventhal S., Hensel A., Petzoldt K., Pabst R. Effects of microbial stimulation on the number, size and activity of bronchus-associated lymphoid tissue (BALT) structures in the pig. Int J Exp Pathol. 1992 Jun;73(3):351–357. [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect Immun. 1986 Nov;54(2):575–582. doi: 10.1128/iai.54.2.575-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I., Olander H. J. Isolation and biological characterization of two lipopolysaccharides and a capsular-enriched polysaccharide preparation from Haemophilus pleuropneumoniae. Am J Vet Res. 1986 Jul;47(7):1433–1441. [PubMed] [Google Scholar]
- Gehrke I., Pabst R. Cell composition and lymphocyte subsets in the bronchoalveolar lavage of normal pigs of different ages in comparison with germfree and pneumonic pigs. Lung. 1990;168(2):79–92. doi: 10.1007/BF02719678. [DOI] [PubMed] [Google Scholar]
- Huang Y. T., Chu R. M., Liu R. S., Weng C. N. Morphologic studies of intrapulmonary airway mucosa-associated lymphoid tissues in swine. Vet Immunol Immunopathol. 1990 May;25(1):13–22. doi: 10.1016/0165-2427(90)90106-3. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Ma J., Workman T., Gogolewski R. P., Anderson P. Virulence properties and protective efficacy of the capsular polymer of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5. Infect Immun. 1988 Aug;56(8):1880–1889. doi: 10.1128/iai.56.8.1880-1889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Purification and partial characterization of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1573–1579. doi: 10.1128/iai.55.7.1573-1579.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Todd J., Koch C., Nicolet J. Serotype specificity of immunological assays for the capsular polymer of Actinobacillus pleuropneumoniae serotypes 1 and 9. Vet Microbiol. 1992 Jun 15;31(4):351–362. doi: 10.1016/0378-1135(92)90127-f. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Virulence properties of Actinobacillus pleuropneumoniae. Microb Pathog. 1991 Nov;11(5):305–316. doi: 10.1016/0882-4010(91)90016-4. [DOI] [PubMed] [Google Scholar]
- Jones P. D., Ada G. L. Influenza-specific antibody-secreting cells and B cell memory in the murine lung after immunization with wild-type, cold-adapted variant and inactivated influenza viruses. Vaccine. 1987 Sep;5(3):244–248. doi: 10.1016/0264-410x(87)90109-5. [DOI] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Leibovitz E. E., Zhao-Ri G. Oral immunization and secretory immunity to viruses. Curr Top Microbiol Immunol. 1989;146:73–81. doi: 10.1007/978-3-642-74529-4_8. [DOI] [PubMed] [Google Scholar]
- Pabst R., Binns R. M. Heterogeneity of lymphocyte homing physiology: several mechanisms operate in the control of migration to lymphoid and non-lymphoid organs in vivo. Immunol Rev. 1989 Apr;108:83–109. doi: 10.1111/j.1600-065x.1989.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Pabst R. Compartmentalization and kinetics of lymphoid cells in the lung. Reg Immunol. 1990 Jan-Feb;3(1):62–71. [PubMed] [Google Scholar]
- Pichichero M. E., Hall C. B., Insel R. A. A mucosal antibody response following systemic Haemophilus influenzae type B infection in children. J Clin Invest. 1981 May;67(5):1482–1489. doi: 10.1172/JCI110178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero M. E., Insel R. A. Relationship between naturally occurring human mucosal and serum antibody to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1982 Aug;146(2):243–248. doi: 10.1093/infdis/146.2.243. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Miniats O. P., Sinclair P. Protective efficacy of capsule extracts of Haemophilus pleuropneumoniae in pigs and mice. Vet Microbiol. 1986 Sep;12(3):229–240. doi: 10.1016/0378-1135(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Lue C., Moldoveanu Z., Kiyono H., McGhee J. R., Prchal J. T., Halpern N. B., Mestecky J. Systemic immunization for the induction of IgA responses. Curr Top Microbiol Immunol. 1989;146:161–168. doi: 10.1007/978-3-642-74529-4_17. [DOI] [PubMed] [Google Scholar]
- Thomson C. M., Chanter N., Wathes C. M. Survival of toxigenic Pasteurella multocida in aerosols and aqueous liquids. Appl Environ Microbiol. 1992 Mar;58(3):932–936. doi: 10.1128/aem.58.3.932-936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udeze F. A., Kadis S. Inhibition of bactericidal activity of anticapsular antibody by nonspecific antibodies reactive with surface-exposed antigenic determinants on Actinobacillus pleuropneumoniae. Infect Immun. 1992 Sep;60(9):3852–3860. doi: 10.1128/iai.60.9.3852-3860.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace F. J., Cripps A. W., Clancy R. L., Husband A. J., Witt C. S. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunology. 1991 Sep;74(1):68–73. [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Grimes S. R., Jr, Lamm M. E. Gut-associated lymphoid tissue as source of an IgA immune response in respiratory tissues after oral immunization and intrabronchial challenge. Cell Immunol. 1987 Apr 15;106(1):132–138. doi: 10.1016/0008-8749(87)90156-0. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]



