Abstract
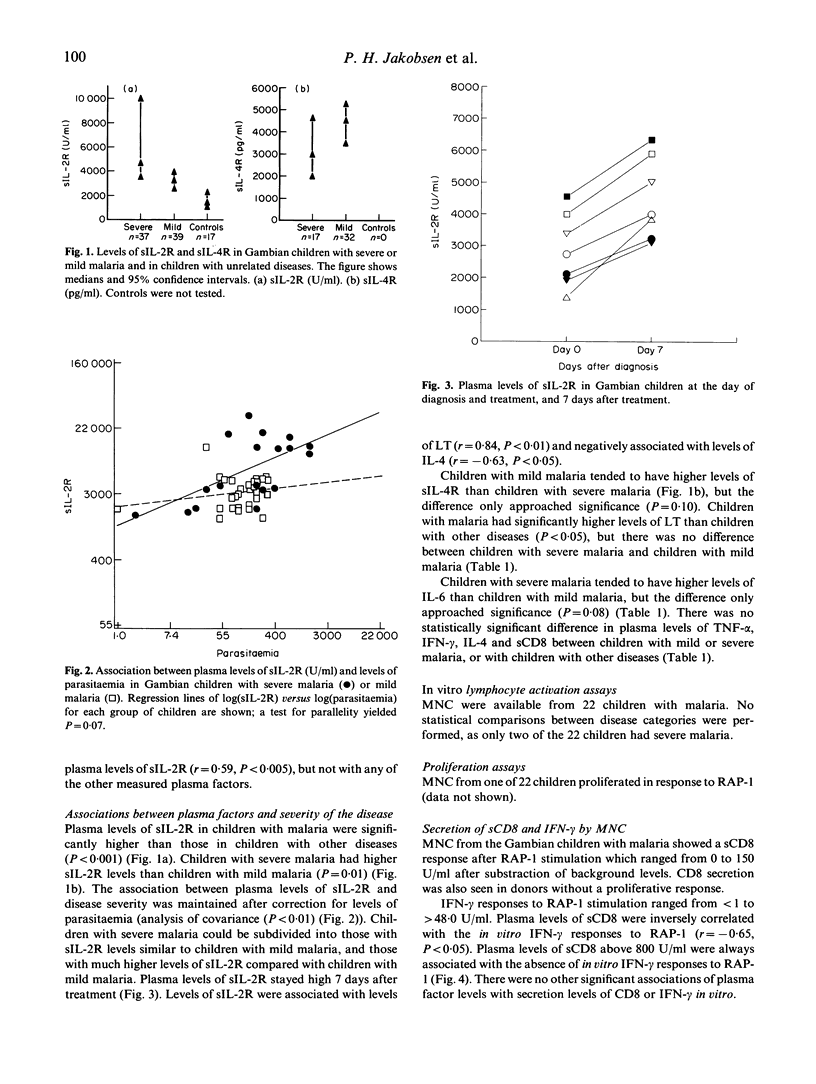
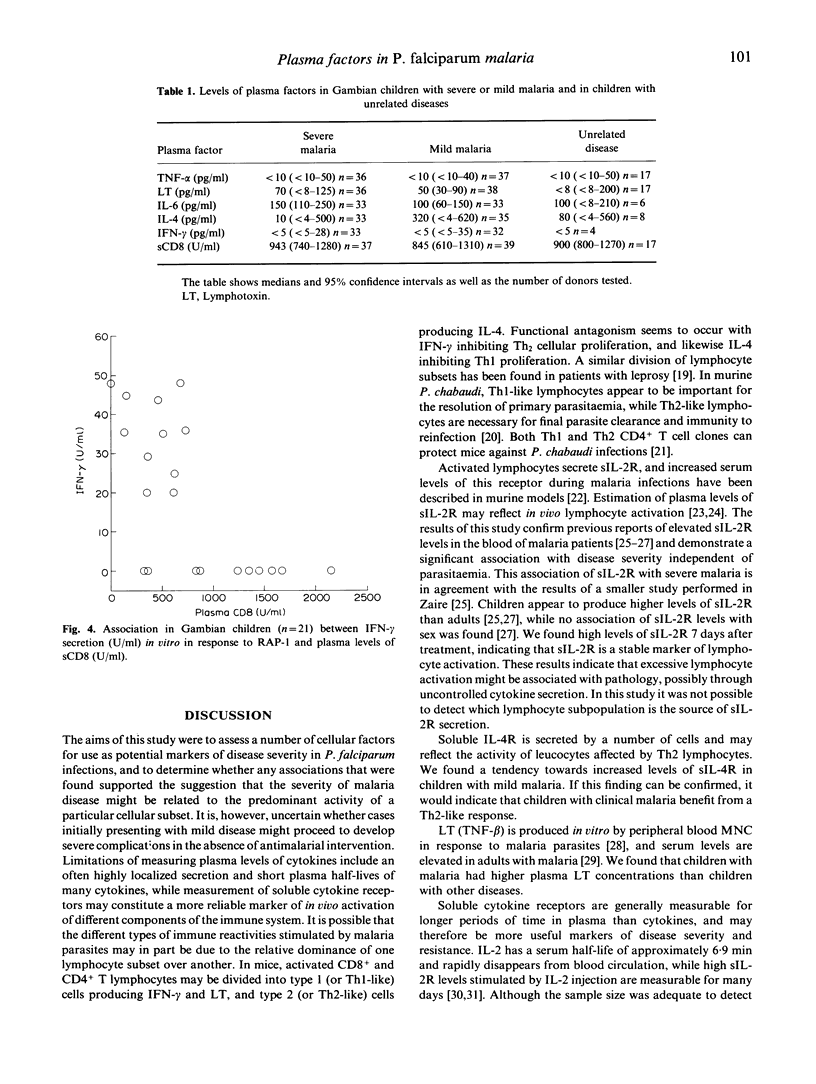
Plasma samples from children with mild and severe Plasmodium falciparum malaria and from children with unrelated diseases were collected to investigate whether the clinical outcome of infection was associated with plasma factors which reflected the activity of different cells of the immune system. Children with severe P. falciparum malaria had significantly higher plasma levels of soluble IL-2R than children with mild malaria. Plasma levels of IL-2R and levels of parasitaemia were significantly correlated. Neither parasitaemia nor plasma levels of tumour necrosis factor-alpha (TNF-alpha), IL-6, lymphotoxin (LT), interferon-gamma (IFN-gamma), IL-4, soluble IL-4R or soluble CD8 differed significantly between the two groups of children with malaria. High plasma levels of soluble CD8 were associated with failure of lymphocytes to produce IFN-gamma in vitro following stimulation with P. falciparum antigen. We conclude that soluble IL-2R is a useful marker of disease severity independently of the association with levels of parasitaemia, and that functional regulation of different lymphocyte subsets occurs during acute malaria episodes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Zeid Y. A., Theander T. G., Abdulhadi N. H., Hviid L., Saeed B. O., Jepsen S., Jensen J. B., Bayoumi R. A. Modulation of the cellular immune response during Plasmodium falciparum infections in sickle cell trait individuals. Clin Exp Immunol. 1992 Apr;88(1):112–118. doi: 10.1111/j.1365-2249.1992.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Salgame P., Diamond B. Revisiting and revising suppressor T cells. Immunol Today. 1992 Apr;13(4):131–136. doi: 10.1016/0167-5699(92)90110-S. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Carlson J., Helmby H., Hill A. V., Brewster D., Greenwood B. M., Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990 Dec 15;336(8729):1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- Chizzolini C., Grau G. E., Geinoz A., Schrijvers D. T lymphocyte interferon-gamma production induced by Plasmodium falciparum antigen is high in recently infected non-immune and low in immune subjects. Clin Exp Immunol. 1990 Jan;79(1):95–99. doi: 10.1111/j.1365-2249.1990.tb05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Gray K. M., Rockett E. J., Cowden W. B., Rockett K. A., Ferrante A., Aggarwal B. B. Increased lymphotoxin in human malarial serum, and the ability of this cytokine to increase plasma interleukin-6 and cause hypoglycaemia in mice: implications for malarial pathology. Trans R Soc Trop Med Hyg. 1992 Nov-Dec;86(6):602–607. doi: 10.1016/0035-9203(92)90144-2. [DOI] [PubMed] [Google Scholar]
- Deloron P., Lepers J. P., Coulanges P. Evolution of the levels of soluble interleukin-2 receptors during Plasmodium falciparum and P. vivax malaria. J Clin Microbiol. 1989 Aug;27(8):1887–1889. doi: 10.1128/jcm.27.8.1887-1889.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Staugas R. E., Rowan-Kelly B., Bresatz S., Kumaratilake L. M., Rzepczyk C. M., Adolf G. R. Production of tumor necrosis factors alpha and beta by human mononuclear leukocytes stimulated with mitogens, bacteria, and malarial parasites. Infect Immun. 1990 Dec;58(12):3996–4003. doi: 10.1128/iai.58.12.3996-4003.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Heremans H., Piguet P. F., Pointaire P., Lambert P. H., Billiau A., Vassalli P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Hansen M. B., Svenson M., Bendtzen K. Human anti-interleukin 1 alpha antibodies. Immunol Lett. 1991 Sep;30(1):133–139. doi: 10.1016/0165-2478(91)90102-g. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Hviid L., Theander T. G., Abu-Zeid Y. A., Abdulhadi N. H., Jakobsen P. H., Saeed B. O., Jepsen S., Bayoumi R. A., Jensen J. B. Loss of cellular immune reactivity during acute Plasmodium falciparum malaria. FEMS Microbiol Immunol. 1991 Aug;3(4):219–227. doi: 10.1111/j.1574-6968.1991.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Hviid L., Theander T. G., Afare E. A., Ridley R. G., Heegaard P. M., Stuber D., Dalsgaard K., Nkrumah F. K. Specific T-cell recognition of the merozoite proteins rhoptry-associated protein 1 and erythrocyte-binding antigen 1 of Plasmodium falciparum. Infect Immun. 1993 Jan;61(1):268–273. doi: 10.1128/iai.61.1.268-273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen P. H., Moon R., Ridley R. G., Bate C. A., Taverne J., Hansen M. B., Takacs B., Playfair J. H., McBride J. S. Tumour necrosis factor and interleukin-6 production induced by components associated with merozoite proteins of Plasmodium falciparum. Parasite Immunol. 1993 Apr;15(4):229–237. doi: 10.1111/j.1365-3024.1993.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Riley E. M., Allen S. J., Larsen S. O., Bennett S., Jepsen S., Greenwood B. M. Differential antibody response of Gambian donors to soluble Plasmodium falciparum antigens. Trans R Soc Trop Med Hyg. 1991 Jan-Feb;85(1):26–32. doi: 10.1016/0035-9203(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Kemp M., Hansen M. B., Theander T. G. Recognition of Leishmania antigens by T lymphocytes from nonexposed individuals. Infect Immun. 1992 Jun;60(6):2246–2251. doi: 10.1128/iai.60.6.2246-2251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsner P. G., Bienzle U. Soluble CD8 antigen in Plasmodium falciparum malaria. J Infect Dis. 1989 Aug;160(2):357–358. doi: 10.1093/infdis/160.2.357. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Cannon J. G., Manogue K. R., Cerami A., Dinarello C. A., Greenwood B. M. Tumour necrosis factor production in Falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989 Sep;77(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Playfair J. H. Serum IL-2 inhibitor in mice. I. Increase during infection. Immunology. 1985 Sep;56(1):113–118. [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Custer M. C., Sharrow S. O., Rubin L. A., Nelson D. L., Rosenberg S. A. In vivo administration of purified human interleukin-2 to patients with cancer: development of interleukin-2 receptor positive cells and circulating soluble interleukin-2 receptors following interleukin-2 administration. Cancer Res. 1987 Apr 15;47(8):2188–2195. [PubMed] [Google Scholar]
- Lotze M. T., Frana L. W., Sharrow S. O., Robb R. J., Rosenberg S. A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 1985 Jan;134(1):157–166. [PubMed] [Google Scholar]
- Marsh K., Otoo L., Hayes R. J., Carson D. C., Greenwood B. M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989 May-Jun;83(3):293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- Mshana R. N., McLean S., Boulandi J. In vitro cell-mediated immune responses to Plasmodium falciparum schizont antigens in adults from a malaria endemic area: CD8+ T lymphocytes inhibit the response of low responder individuals. Int Immunol. 1990;2(12):1121–1132. doi: 10.1093/intimm/2.12.1121. [DOI] [PubMed] [Google Scholar]
- Nguyen-Dinh P., Greenberg A. E. Increased levels of released interleukin-2 receptors in Plasmodium falciparum malaria. J Infect Dis. 1988 Dec;158(6):1403–1404. doi: 10.1093/infdis/158.6.1403. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Allen S. J., Wheeler J. G., Blackman M. J., Bennett S., Takacs B., Schönfeld H. J., Holder A. A., Greenwood B. M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992 May;14(3):321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Jakobsen P. H., Allen S. J., Wheeler J. G., Bennett S., Jepsen S., Greenwood B. M. Immune response to soluble exoantigens of Plasmodium falciparum may contribute to both pathogenesis and protection in clinical malaria: evidence from a longitudinal, prospective study of semi-immune African children. Eur J Immunol. 1991 Apr;21(4):1019–1025. doi: 10.1002/eji.1830210424. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Jobe O., Whittle H. C. CD8+ T cells inhibit Plasmodium falciparum-induced lymphoproliferation and gamma interferon production in cell preparations from some malaria-immune individuals. Infect Immun. 1989 Apr;57(4):1281–1284. doi: 10.1128/iai.57.4.1281-1284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E. M., Rowe P., Allen S. J., Greenwood B. M. Soluble plasma IL-2 receptors and malaria. Clin Exp Immunol. 1993 Mar;91(3):495–499. doi: 10.1111/j.1365-2249.1993.tb05930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S. Functional characterization of protective CD4+ T-cell clones reactive to the murine malaria parasite Plasmodium chabaudi. Immunology. 1992 Sep;77(1):99–105. [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S., Severn A., Moncada S., Liew F. Y. The role of TH1 and TH2 cells in a rodent malaria infection. Science. 1993 Jun 25;260(5116):1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]



