Abstract
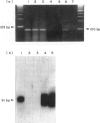
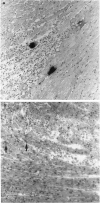
Following infection with Coxsackievirus B3 (CVB3), A-strain mice develop ongoing myocarditis that persists after the virus ceases to be cultivatable from heart tissue. We studied the natural history of this virus-induced but apparently autoimmune inflammation by means of in situ hybridization (ISH) and by polymerase chain reaction (PCR). Both ISH and culture allowed detection of virus up to 2 weeks post-infection in virtually all heart tissues. In contrast, PCR revealed the presence of viral genome for a substantially longer period of time, i.e. at least 34 days after CVB3 infection. Similarly, the majority of mice showed myocardial inflammation at this time point. However, the persistence of virus did not correlate with ongoing myocarditis, and vice versa. Most mice with ongoing myocarditis produced heart myosin autoantibodies, most probably as a result of tissue damage. The lack of correlation between presence of ongoing inflammation and persistence of virus supports our previous view that the late phase of CVB3-induced myocarditis is mediated by autoimmunological mechanisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez F. L., Neu N., Rose N. R., Craig S. W., Beisel K. W. Heart-specific autoantibodies induced by Coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987 Apr;43(1):129–139. doi: 10.1016/0090-1229(87)90164-4. [DOI] [PubMed] [Google Scholar]
- Archard L. C., Bowles N. E., Cunningham L., Freeke C. A., Olsen E. G., Rose M. L., Meany B., Why H. J., Richardson P. J. Molecular probes for detection of persisting enterovirus infection of human heart and their prognostic value. Eur Heart J. 1991 Aug;12 (Suppl 500):56–59. doi: 10.1093/eurheartj/12.suppl_d.56. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Tracy S., Gauntt C. J., Fortmueller U. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990 May;28(5):843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Huber S. A., Sriram S. Animal models of picornavirus-induced autoimmune disease: their possible relevance to human disease. Lab Invest. 1990 Oct;63(4):432–446. [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham L., Bowles N. E., Lane R. J., Dubowitz V., Archard L. C. Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol. 1990 Jun;71(Pt 6):1399–1402. doi: 10.1099/0022-1317-71-6-1399. [DOI] [PubMed] [Google Scholar]
- Cunningham M. W., Antone S. M., Gulizia J. M., McManus B. M., Fischetti V. A., Gauntt C. J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefler H., Childers H., Montminy M. R., Lechan R. M., Goodman R. H., Wolfe H. J. In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J. 1986 Nov-Dec;18(11-12):597–604. doi: 10.1007/BF01675295. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Klingel K., Mertsching H., Canu A., Hohenadl C., Zell R., Reimann B. Y., Heim A., McManus B. M., Foulis A. K. Molecular studies on enteroviral heart disease: patterns of acute and persistent infections. Eur Heart J. 1991 Aug;12 (Suppl 500):49–55. doi: 10.1093/eurheartj/12.suppl_d.49. [DOI] [PubMed] [Google Scholar]
- Keeling P. J., Jeffery S., Caforio A. L., Taylor R., Bottazzo G. F., Davies M. J., McKenna W. J. Similar prevalence of enteroviral genome within the myocardium from patients with idiopathic dilated cardiomyopathy and controls by the polymerase chain reaction. Br Heart J. 1992 Dec;68(6):554–559. doi: 10.1136/hrt.68.12.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyu B., Matsumori A., Sato Y., Okada I., Chapman N. M., Tracy S. Cardiac persistence of cardioviral RNA detected by polymerase chain reaction in a murine model of dilated cardiomyopathy. Circulation. 1992 Aug;86(2):522–530. doi: 10.1161/01.cir.86.2.522. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- Neu N., Craig S. W., Rose N. R., Alvarez F., Beisel K. W. Coxsackievirus induced myocarditis in mice: cardiac myosin autoantibodies do not cross-react with the virus. Clin Exp Immunol. 1987 Sep;69(3):566–574. [PMC free article] [PubMed] [Google Scholar]
- Neu N., Ploier B. Experimentally-induced autoimmune myocarditis: production of heart myosin-specific autoantibodies within the inflammatory infiltrate. Autoimmunity. 1991;8(4):317–322. doi: 10.3109/08916939109007639. [DOI] [PubMed] [Google Scholar]
- Neu N., Ploier B., Ofner C. Cardiac myosin-induced myocarditis. Heart autoantibodies are not involved in the induction of the disease. J Immunol. 1990 Dec 15;145(12):4094–4100. [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Okada I., Matsumori A., Kawai C., Yodoi J., Tracy S. The viral genome in experimental murine Coxsackievirus B3 myocarditis: a Northern blotting analysis. J Mol Cell Cardiol. 1990 Sep;22(9):999–1008. doi: 10.1016/0022-2828(90)91039-a. [DOI] [PubMed] [Google Scholar]
- Rose N. R., Herskowitz A., Neumann D. A., Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988 Apr;9(4):117–120. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- Smith S. C., Allen P. M. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991 Oct 1;147(7):2141–2147. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tracy S., Wiegand V., McManus B., Gauntt C., Pallansch M., Beck M., Chapman N. Molecular approaches to enteroviral diagnosis in idiopathic cardiomyopathy and myocarditis. J Am Coll Cardiol. 1990 Jun;15(7):1688–1694. doi: 10.1016/0735-1097(90)92846-t. [DOI] [PubMed] [Google Scholar]
- Wee L., Liu P., Penn L., Butany J. W., McLaughlin P. R., Sole M. J., Liew C. C. Persistence of viral genome into late stages of murine myocarditis detected by polymerase chain reaction. Circulation. 1992 Nov;86(5):1605–1614. doi: 10.1161/01.cir.86.5.1605. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]