Abstract
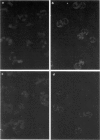
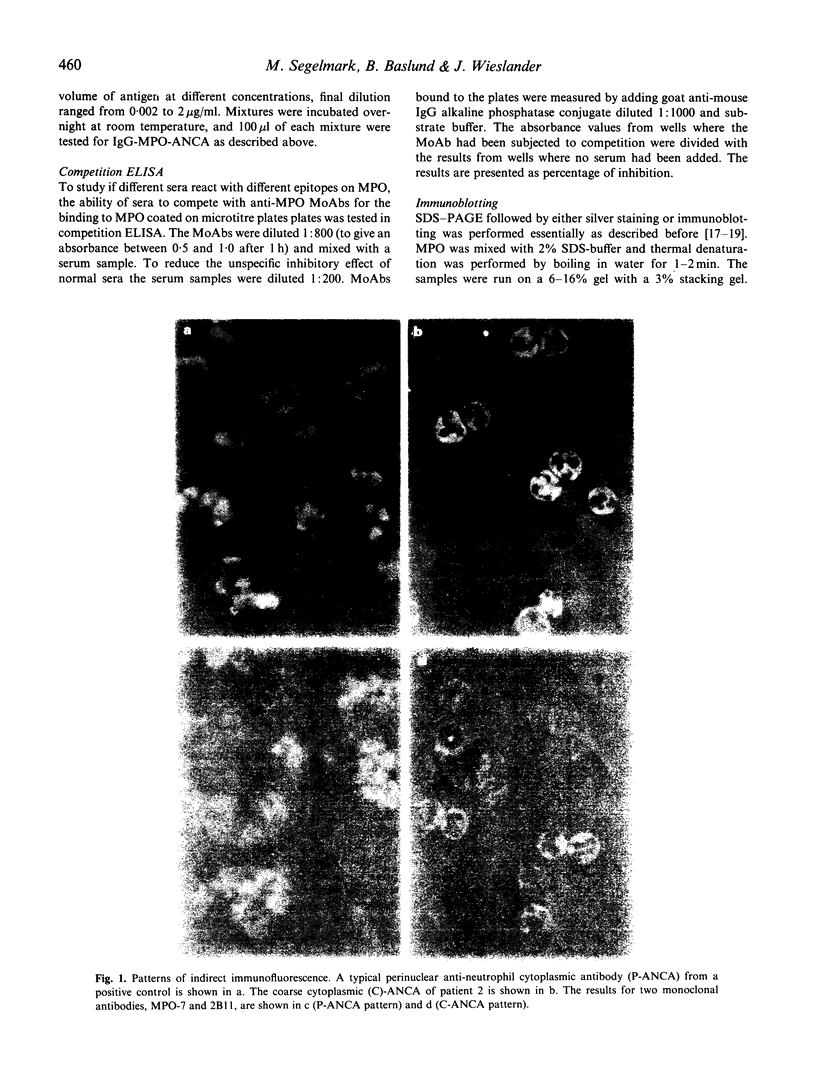
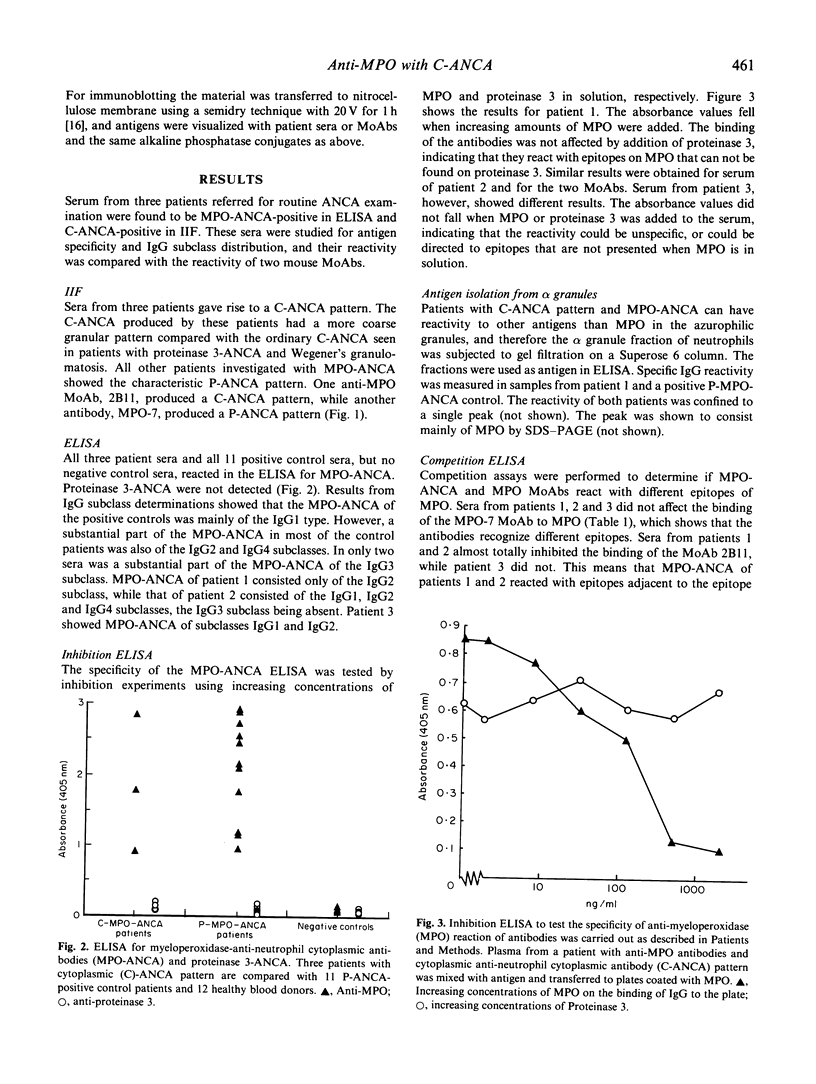
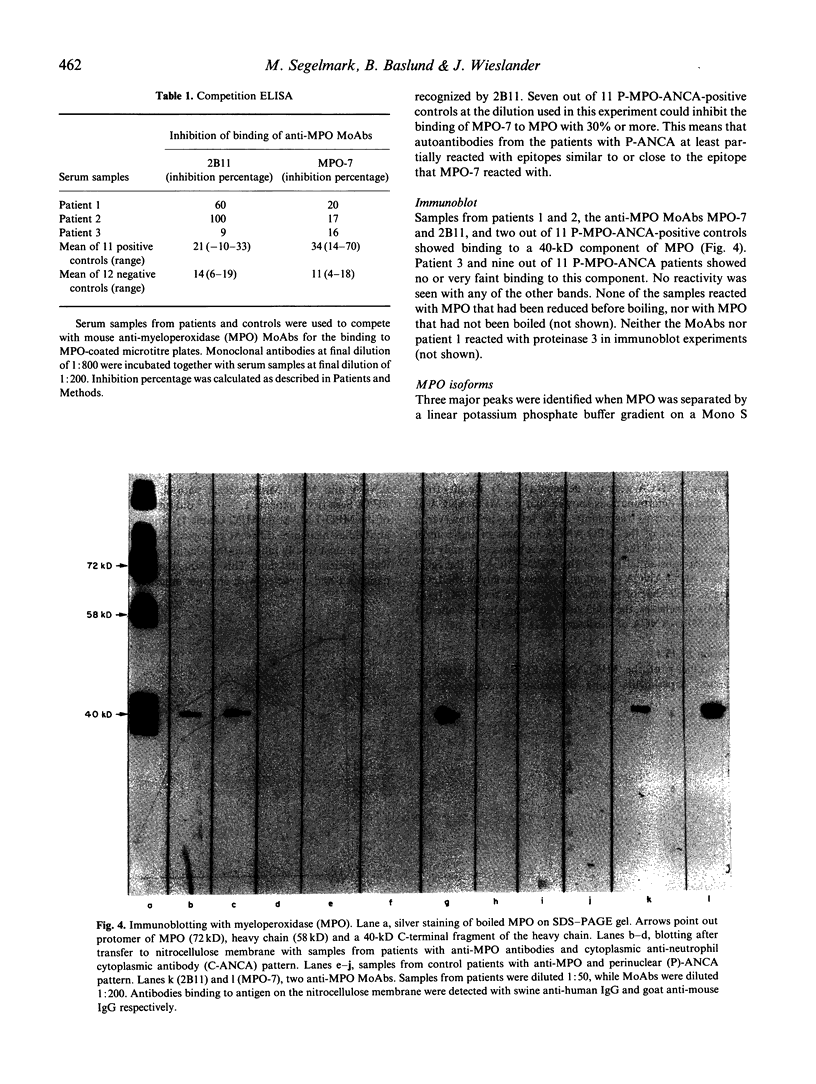
Rapidly progressive glomerulonephritis with or without other signs of systemic vasculitis is often accompanied by antibodies to myeloperoxidase. Such antibodies normally produce a perinuclear pattern on ethanol-fixed neutrophils (perinuclear anti-neutrophil cytoplasm antibodies (P-ANCA)) at indirect immunofluorescence. We report here sera from three patients that are anti-myeloperoxidase-positive in ELISA that instead produce a cytoplasmic pattern (classical anti-neutrophil cytoplasmic antibodies (C-ANCA)), a pattern normally seen in conjunction with antibodies to proteinase 3. These sera did not react with proteinase 3. For two of the sera the specificity of the anti-myeloperoxidase reaction was confirmed with inhibition-ELISA experiments and with immunoblotting. A mouse anti-myeloperoxidase MoAb that produces a cytoplasmic pattern is also described. Competition ELISA experiments show that this antibody and anti-myeloperoxidase sera with cytoplasmic pattern recognize epitopes that are separate from epitopes recognized by another perinuclear pattern producing anti-myeloperoxidase MoAb. 'Cytoplasmic pattern' epitopes as well as 'perinuclear pattern' epitopes can be found on all three major myeloperoxidase isoforms, after separation by ion exchange chromatography. Affinity chromatography, using the cytoplasmic pattern producing anti-myeloperoxidase monoclonal antibody, shows that the epitope recognized by this MoAb is present on all myeloperoxidase molecules. This epitope is not confined to any special subpopulation. These findings indicate that all myeloperoxidase do not relocate after ethanol fixation, and that C-ANCA and P-ANCA epitopes exist simultaneously on the same myeloperoxidase molecule. We propose that the two immunofluorescence patterns arise due to different availabilities of the epitopes in the microenvironment where myeloperoxidase is present.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Charles L. A., Falk R. J., Jennette J. C. Reactivity of anti-neutrophil cytoplasmic autoantibodies with HL-60 cells. Clin Immunol Immunopathol. 1989 Nov;53(2 Pt 1):243–253. doi: 10.1016/0090-1229(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Esnault V. L., Jayne D. R., Keogan M. T., Brownlee A. A., Testa A., Lecarrer D., Brown D. L., Lockwood C. M. Anti-neutrophil cytoplasm antibodies in patients with monoclonal gammopathies. J Clin Lab Immunol. 1990 Aug;32(4):153–159. [PubMed] [Google Scholar]
- Falk R. J. ANCA-associated renal disease. Kidney Int. 1990 Nov;38(5):998–1010. doi: 10.1038/ki.1990.304. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Becker M., Terrell R., Jennette J. C. Anti-myeloperoxidase autoantibodies react with native but not denatured myeloperoxidase. Clin Exp Immunol. 1992 Aug;89(2):274–278. doi: 10.1111/j.1365-2249.1992.tb06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R. J., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988 Jun 23;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Gratadour P., Fouque D., Laville M., Fourcade J., Ffrench M., Colon S., Berger F., Loire R., Cordier J. F., Zech P. Wegener's granulomatosis with antiproteinase-3 antibodies occurring after Hodgkin's disease. Nephron. 1993;64(3):456–461. doi: 10.1159/000187371. [DOI] [PubMed] [Google Scholar]
- Gross W. L., Schmitt W. H., Csernok E. ANCA and associated diseases: immunodiagnostic and pathogenetic aspects. Clin Exp Immunol. 1993 Jan;91(1):1–12. doi: 10.1111/j.1365-2249.1993.tb03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashinaka K., Nishio C., Hur S. J., Sakiyama F., Tsunasawa S., Yamada M. Multiple species of myeloperoxidase messenger RNAs produced by alternative splicing and differential polyadenylation. Biochemistry. 1988 Aug 9;27(16):5906–5914. doi: 10.1021/bi00416a013. [DOI] [PubMed] [Google Scholar]
- Johansson C., Butkowski R., Wieslander J. Characterization of monoclonal antibodies to the globular domain of collagen IV. Connect Tissue Res. 1991;25(3-4):229–241. doi: 10.3109/03008209109029159. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüdemann J., Utecht B., Gross W. L. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990 Jan 1;171(1):357–362. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M., Malech H. L. Analysis of the peptide subunits of human neutrophil myeloperoxidase. Blood. 1986 May;67(5):1504–1507. [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Segelmark M., Wieslander J. IgG subclasses of antineutrophil cytoplasm autoantibodies (ANCA). Nephrol Dial Transplant. 1993;8(8):696–702. doi: 10.1093/ndt/8.8.696. [DOI] [PubMed] [Google Scholar]
- Taylor K. L., Guzman G. S., Pohl J., Kinkade J. M., Jr Distinct chromatographic forms of human hemi-myeloperoxidase obtained by reductive cleavage of the dimeric enzyme. Evidence for subunit heterogeneity. J Biol Chem. 1990 Sep 15;265(26):15938–15946. [PubMed] [Google Scholar]
- Taylor K. L., Pohl J., Kinkade J. M., Jr Unique autolytic cleavage of human myeloperoxidase. Implications for the involvement of active site MET409. J Biol Chem. 1992 Dec 15;267(35):25282–25288. [PubMed] [Google Scholar]
- Wiik A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS Suppl. 1989;6:12–13. [PubMed] [Google Scholar]
- van der Woude F. J., Rasmussen N., Lobatto S., Wiik A., Permin H., van Es L. A., van der Giessen M., van der Hem G. K., The T. H. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985 Feb 23;1(8426):425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]