Abstract
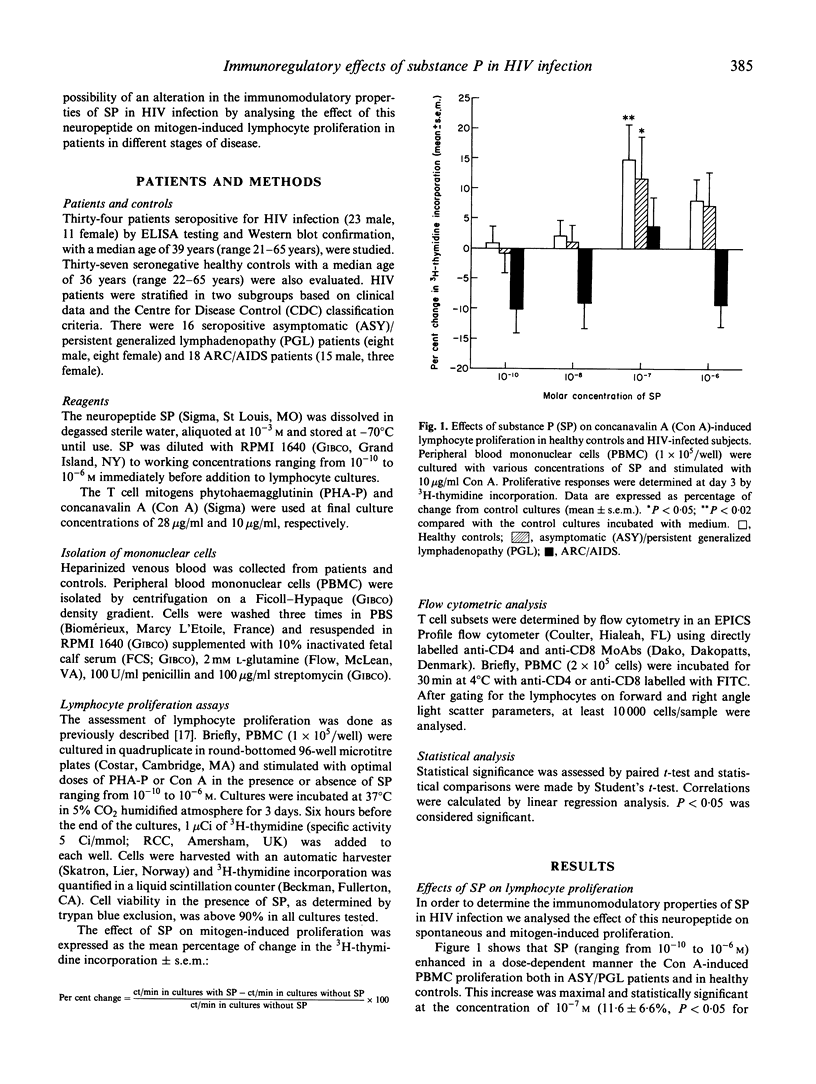
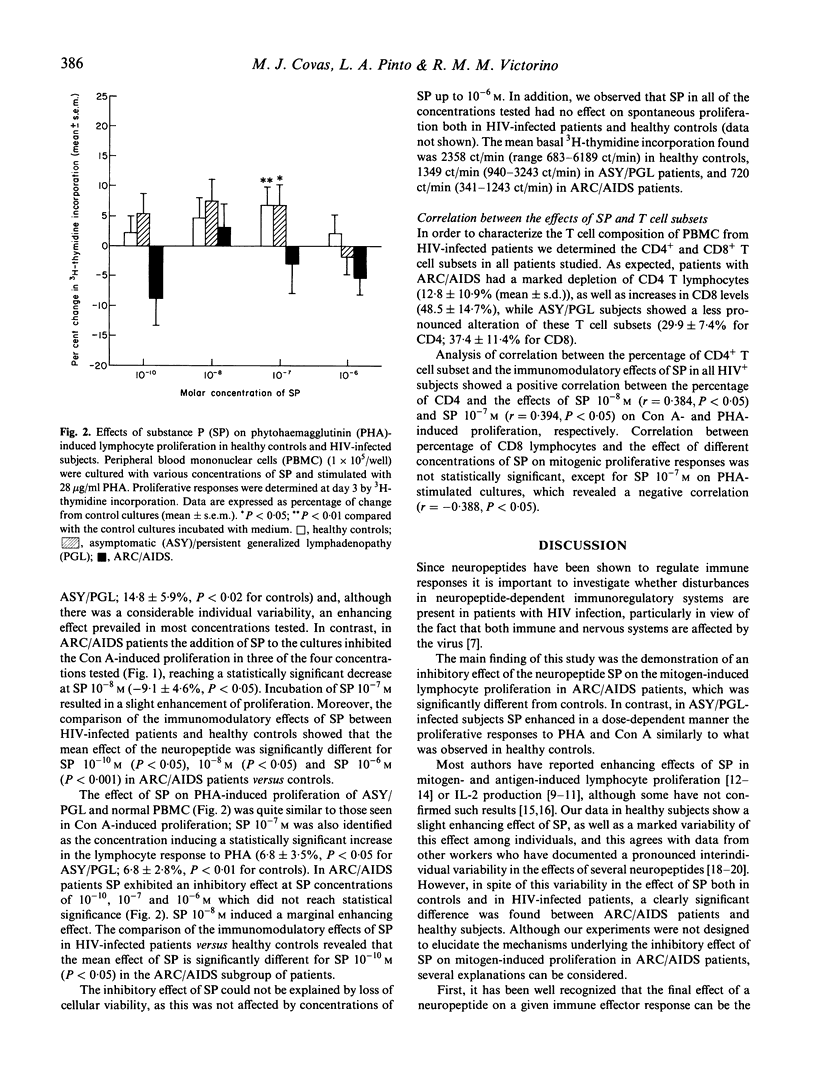
The neuropeptide substance P (SP) is known to increase cell-mediated immune responses in animal models and healthy subjects. Several studies have suggested an involvement of neuropeptides in the immunopathogenesis of some diseases. The study of the immunomodulatory effects of neuropeptides, namely SP, may represent a model for the analysis of immunoregulatory defects in HIV infection at the level of the interaction between the immune and nervous systems, both of which are known to be affected by the virus. In the present study, we investigate the possibility of a disturbance in the immunomodulatory properties of SP in HIV infection by analysing the effects of SP (10(-10)-10(-6) M) on the lymphocyte proliferative responses to concanavalin A (Con A) and phytohaemagglutinin (PHA) assessed by 3H-thymidine incorporation in peripheral blood lymphocytes from 34 HIV-infected patients (16 asymptomatic (ASY)/persistent generalized lymphadenopathy (PGL); 18 ARC/AIDS) and in 37 healthy subjects. In ASY/PGL HIV-infected patients, SP 10(-7) M was identified as the concentration inducing the maximal increase in the lymphocyte responses to Con A and PHA, similarly to what was observed in healthy subjects. In ARC/AIDS patients, SP appeared to inhibit the mitogenic responses, particularly those induced by Con A, in contrast to the effects found either in healthy subjects or in ASY/PGL patients. These results suggest the existence of an alteration in the in vitro immunomodulatory properties of SP in ARC/AIDS patients compared with healthy subjects and ASY/PGL patients. In conclusion, the unexpected finding of an inhibitory effect of SP on lymphocyte proliferation from ARC/AIDS patients justifies further investigation of the neuropeptide-dependent immunoregulatory systems in HIV infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzari C., Rossi M. E., Resti M., Caldini A. L., Lega L., Galli L., Fico E., Vierucci A. Alterati livelli di sostanza P e somatostatina nei bambini HIV positivi. Pediatr Med Chir. 1992 Nov-Dec;14(6):577–581. [PubMed] [Google Scholar]
- Blalock J. E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989 Jan;69(1):1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- Blazquez M. V., Madueño J. A., Gonzalez R., Jurado R., Bachovchin W. W., Peña J., Muñoz E. Selective decrease of CD26 expression in T cells from HIV-1-infected individuals. J Immunol. 1992 Nov 1;149(9):3073–3077. [PubMed] [Google Scholar]
- Blum A. M., Metwali A., Cook G., Mathew R. C., Elliott D., Weinstock J. V. Substance P modulates antigen-induced, IFN-gamma production in murine Schistosomiasis mansoni. J Immunol. 1993 Jul 1;151(1):225–233. [PubMed] [Google Scholar]
- Brummitt C. F., Sharp B. M., Gekker G., Keane W. F., Peterson P. K. Modulatory effects of beta-endorphin on interferon-gamma production by cultured peripheral blood mononuclear cells: heterogeneity among donors and the influence of culture medium. Brain Behav Immun. 1988 Sep;2(3):187–197. doi: 10.1016/0889-1591(88)90021-9. [DOI] [PubMed] [Google Scholar]
- Calvo C. F., Chavanel G., Senik A. Substance P enhances IL-2 expression in activated human T cells. J Immunol. 1992 Jun 1;148(11):3498–3504. [PubMed] [Google Scholar]
- Fernández-Cruz E., Gelpi E., Longo N., González B., de la Morena M. T., Montes M. G., Roselló J., Ramis I., Suarez A., Fernández A. Increased synthesis and production of prostaglandin E2 by monocytes from drug addicts with AIDS. AIDS. 1989 Feb;3(2):91–96. doi: 10.1097/00002030-198902000-00007. [DOI] [PubMed] [Google Scholar]
- Foley P., Kazazi F., Biti R., Sorrell T. C., Cunningham A. L. HIV infection of monocytes inhibits the T-lymphocyte proliferative response to recall antigens, via production of eicosanoids. Immunology. 1992 Mar;75(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- Heijnen C. J., Zijlstra J., Kavelaars A., Croiset G., Ballieux R. E. Modulation of the immune response by POMC-derived peptides. I. Influence on proliferation of human lymphocytes. Brain Behav Immun. 1987 Dec;1(4):284–291. doi: 10.1016/0889-1591(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Downs M. O., Pontzer C. H. Neuroendocrine peptide hormone regulation of immunity. Chem Immunol. 1992;52:49–83. [PubMed] [Google Scholar]
- Kavelaars A., Jeurissen F., von Frijtag Drabbe Künzel J., Herman van Roijen J., Rijkers G. T., Heijnen C. J. Substance P induces a rise in intracellular calcium concentration in human T lymphocytes in vitro: evidence of a receptor-independent mechanism. J Neuroimmunol. 1993 Jan;42(1):61–70. doi: 10.1016/0165-5728(93)90213-i. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Clark R., Devor M., Helms C., Moskowitz M. A., Basbaum A. I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov 2;226(4674):547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Chen I. S. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991 Jul;5(10):2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Millar D. B., Hough C. J., Mazorow D. L., Gootenberg J. E. Beta-endorphin's modulation of lymphocyte proliferation is dose, donor, and time dependent. Brain Behav Immun. 1990 Sep;4(3):232–242. doi: 10.1016/0889-1591(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Pernow B., Fischer G. H., Folkers K. Presence of substance P-like immunoreactivity in plasma from man and dog. Acta Physiol Scand. 1975 Aug;94(4):542–544. doi: 10.1111/j.1748-1716.1975.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Nio D. A., Moylan R. N., Roche J. K. Modulation of T lymphocyte function by neuropeptides. Evidence for their role as local immunoregulatory elements. J Immunol. 1993 Jun 15;150(12):5281–5288. [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Fauci A. S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993 Feb 4;328(5):327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Pascual D. W., Bost K. L. Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology. 1990 Sep;71(1):52–56. [PMC free article] [PubMed] [Google Scholar]
- Payan D. G., Brewster D. R., Goetzl E. J. Specific stimulation of human T lymphocytes by substance P. J Immunol. 1983 Oct;131(4):1613–1615. [PubMed] [Google Scholar]
- Payan D. G., Brewster D. R., Missirian-Bastian A., Goetzl E. J. Substance P recognition by a subset of human T lymphocytes. J Clin Invest. 1984 Oct;74(4):1532–1539. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Peterson P. K., Sharp B., Gekker G., Brummitt C., Keane W. F. Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J Clin Invest. 1987 Sep;80(3):824–831. doi: 10.1172/JCI113140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P., Gascon P., Ganea D. Immunoregulatory effects of neuropeptides. Stimulation of interleukin-2 production by substance p. J Neuroimmunol. 1992 Mar;37(1-2):65–74. doi: 10.1016/0165-5728(92)90156-f. [DOI] [PubMed] [Google Scholar]
- Roberts A. I., Taunk J., Ebert E. C. Human lymphocytes lack substance P receptors. Cell Immunol. 1992 May;141(2):457–465. doi: 10.1016/0008-8749(92)90163-j. [DOI] [PubMed] [Google Scholar]
- Scicchitano R., Bienenstock J., Stanisz A. M. The differential effect with time of neuropeptides on the proliferative responses of murine Peyer's patch and splenic lymphocytes. Brain Behav Immun. 1987 Sep;1(3):231–237. doi: 10.1016/0889-1591(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Scicchitano R., Biennenstock J., Stanisz A. M. In vivo immunomodulation by the neuropeptide substance P. Immunology. 1988 Apr;63(4):733–735. [PMC free article] [PubMed] [Google Scholar]
- Ulmer A. J., Mattern T., Feller A. C., Heymann E., Flad H. D. CD26 antigen is a surface dipeptidyl peptidase IV (DPPIV) as characterized by monoclonal antibodies clone TII-19-4-7 and 4EL1C7. Scand J Immunol. 1990 Apr;31(4):429–435. doi: 10.1111/j.1365-3083.1990.tb02789.x. [DOI] [PubMed] [Google Scholar]
- Vanham G., Kestens L., De Meester I., Vingerhoets J., Penne G., Vanhoof G., Scharpé S., Heyligen H., Bosmans E., Ceuppens J. L. Decreased expression of the memory marker CD26 on both CD4+ and CD8+ T lymphocytes of HIV-infected subjects. J Acquir Immune Defic Syndr. 1993 Jul;6(7):749–757. [PubMed] [Google Scholar]
- Victorino R. M., Maria V. A., Pinto L. A. Evidence for prostaglandin-producing suppressor cells in drug-induced liver injury and implications in the diagnosis of drug sensitization. Clin Exp Immunol. 1992 Jan;87(1):132–137. doi: 10.1111/j.1365-2249.1992.tb06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Ahmad S., Benter I. F., Chow A., Mizutani S., Ward P. E. Differential processing of substance P and neurokinin A by plasma dipeptidyl(amino)peptidase IV, aminopeptidase M and angiotensin converting enzyme. Peptides. 1991 Nov-Dec;12(6):1357–1364. doi: 10.1016/0196-9781(91)90220-j. [DOI] [PubMed] [Google Scholar]
- Zhu L. P., Chen D., Zhang S. Z., Liu S. L. Observation of the effect of substance P on human T and B lymphocyte proliferation. Immunol Commun. 1984;13(5):457–464. [PubMed] [Google Scholar]



