Abstract
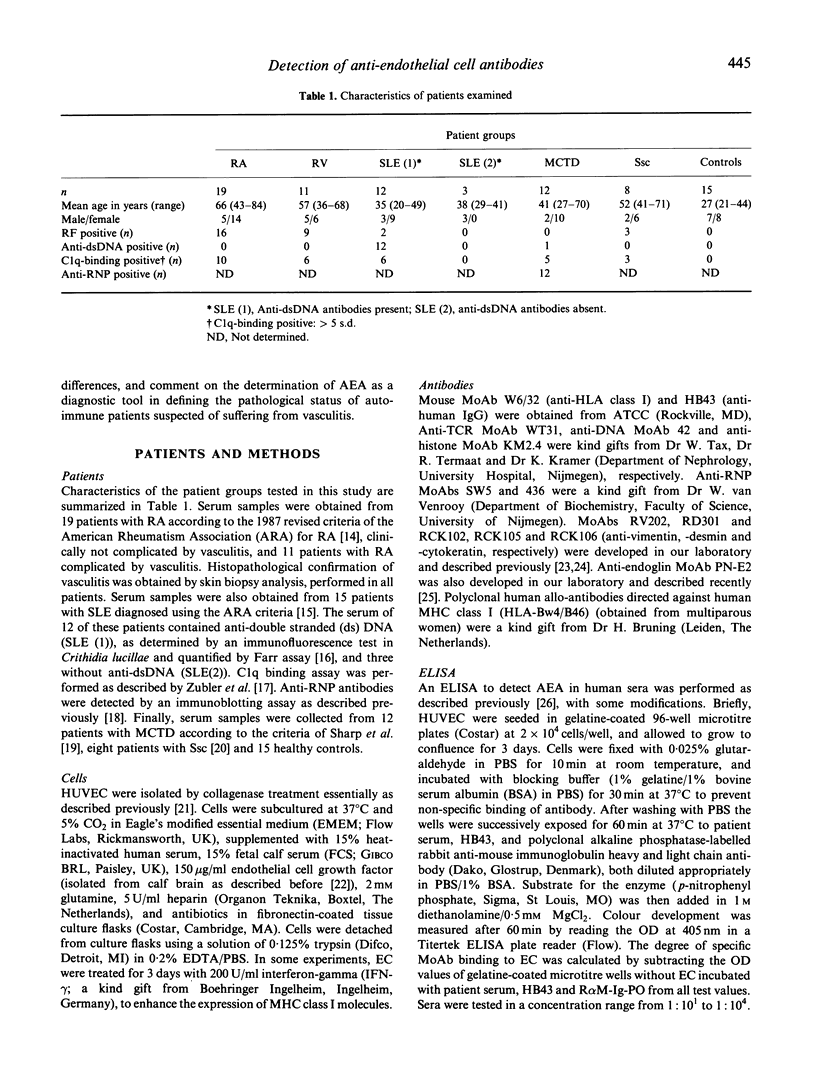
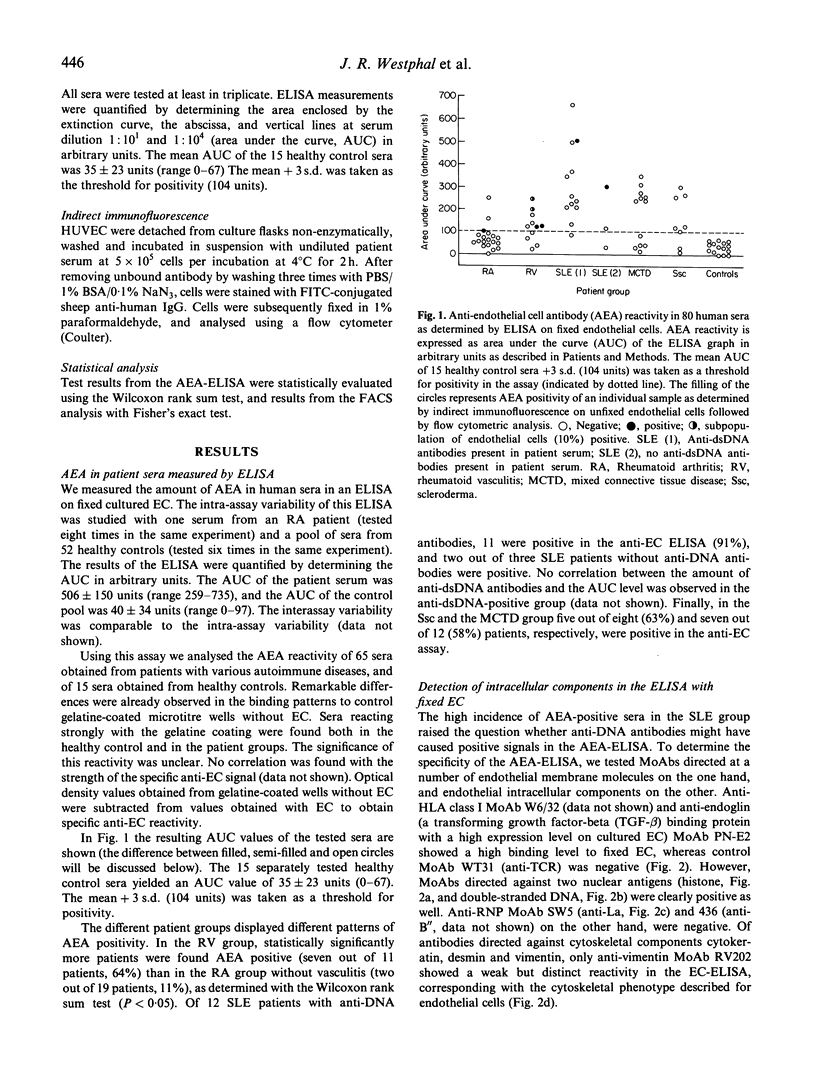
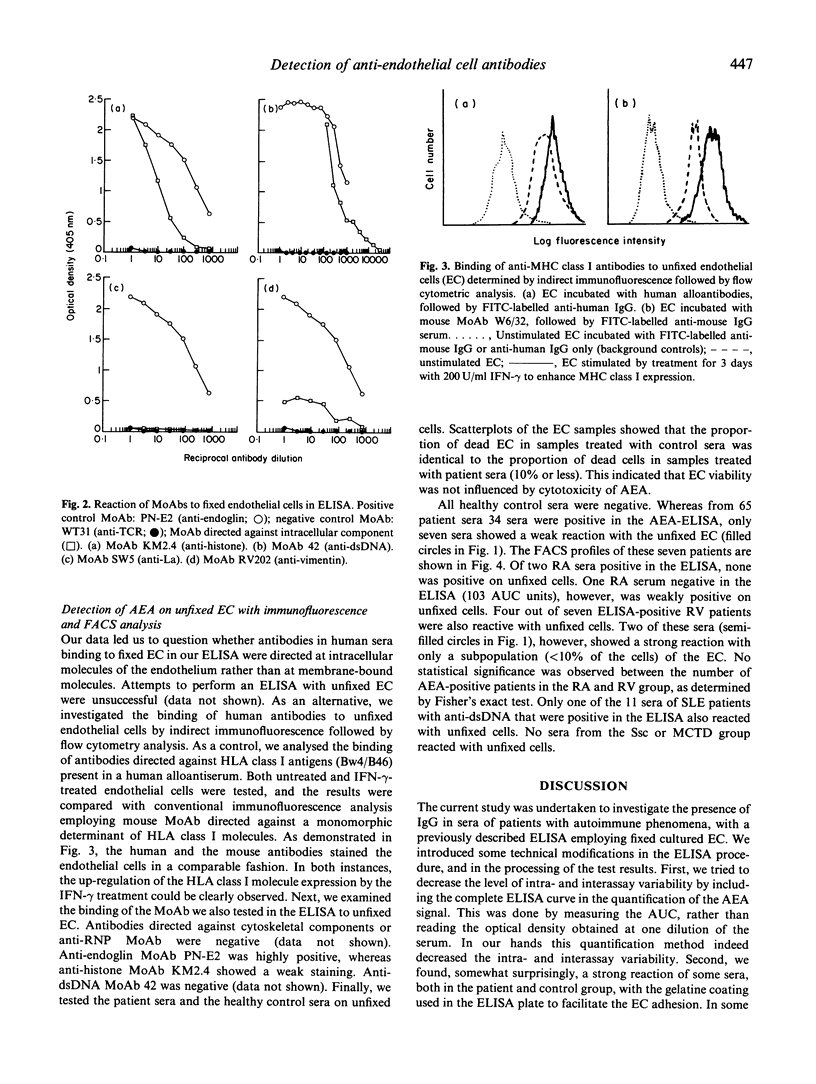
In some patients suffering from rheumatoid arthritis (RA), vasculitis is a clear clinical manifestation, mentioned as rheumatoid vasculitis (RV). Autoantibodies directed against endothelial cells (AEA) have been implicated in the pathogenesis of this disorder, and it has been suggested in a number of studies that testing for AEA should be included in diagnosing RV. To test this hypothesis, we have evaluated the presence of AEA in sera of patients suffering from various autoimmune diseases, employing an ELISA with fixed cultured endothelial cells (EC). In all the groups of patients ELISA-positive sera were present. A significant difference in percentage of positivity was found between the RA and RV group (P < 0.05). In addition, our results indicated that not only antibodies directed against antigens on the EC membrane were detected, but also antibodies directed against intracellular components like DNA, histones and cytoskeletal components. Therefore, we also tested all these patient sera on unfixed intact EC using indirect immunofluorescence followed by FACS analysis. Whereas in the total patient population 34 out of 65 patients were AEA-positive as determined in the ELISA, only seven patients were weakly positive when examined by flow cytometry. We conclude that: (i) an ELISA on fixed EC does not specifically detect AEA. A positive test result is, however, to some extent correlated with the occurrence of vasculitis, and may therefore be helpful in diagnosing this disease; (ii) FACS analysis is a more suitable method than ELISA to measure the presence of membrane-specific AEA in patient sera; (iii) specific IgG-AEA are less common in patients suffering from autoimmune disorders than was assumed previously.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Baguley E., Hughes G. R. Lytic IgG anti-endothelial cell antibodies in vasculitis. Lancet. 1988 Oct 15;2(8616):907–907. doi: 10.1016/s0140-6736(88)92505-6. [DOI] [PubMed] [Google Scholar]
- Brasile L., Kremer J. M., Clarke J. L., Cerilli J. Identification of an autoantibody to vascular endothelial cell-specific antigens in patients with systemic vasculitis. Am J Med. 1989 Jul;87(1):74–80. doi: 10.1016/s0002-9343(89)80486-3. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Heurkens A. H., Lafeber G. J., van Hinsbergh V. W., Cats A. Immune complexes in sera from patients with rheumatoid vasculitis induce polymorphonuclear cell-mediated injury to endothelial cells. Clin Immunol Immunopathol. 1988 Aug;48(2):202–213. doi: 10.1016/0090-1229(88)90084-0. [DOI] [PubMed] [Google Scholar]
- Broers J. L., Carney D. N., Klein Rot M., Schaart G., Lane E. B., Vooijs G. P., Ramaekers F. C. Intermediate filament proteins in classic and variant types of small cell lung carcinoma cell lines: a biochemical and immunochemical analysis using a panel of monoclonal and polyclonal antibodies. J Cell Sci. 1986 Jul;83:37–60. doi: 10.1242/jcs.83.1.37. [DOI] [PubMed] [Google Scholar]
- Broers J. L., Ramaekers F. C., Rot M. K., Oostendorp T., Huysmans A., van Muijen G. N., Wagenaar S. S., Vooijs G. P. Cytokeratins in different types of human lung cancer as monitored by chain-specific monoclonal antibodies. Cancer Res. 1988 Jun 1;48(11):3221–3229. [PubMed] [Google Scholar]
- Chan T. M., Frampton G., Cameron J. S. Identification of DNA-binding proteins on human umbilical vein endothelial cell plasma membrane. Clin Exp Immunol. 1993 Jan;91(1):110–114. doi: 10.1111/j.1365-2249.1993.tb03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Frampton G., Staines N. A., Hobby P., Perry G. J., Cameron J. S. Different mechanisms by which anti-DNA MoAbs bind to human endothelial cells and glomerular mesangial cells. Clin Exp Immunol. 1992 Apr;88(1):68–74. doi: 10.1111/j.1365-2249.1992.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Reeber M., Bina M., DeHoratius R. J. Presence of complement-fixing anti-endothelial cell antibodies in systemic lupus erythematosus. J Clin Invest. 1984 Mar;73(3):611–625. doi: 10.1172/JCI111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz D. P., Houssiau F. A., Ramirez G., Baguley E., McCutcheon J., Vianna J., Haga H. J., Swana G. T., Khamashta M. A., Taylor J. C. Antibodies to endothelial cells in systemic lupus erythematosus: a potential marker for nephritis and vasculitis. Clin Exp Immunol. 1991 Aug;85(2):254–261. doi: 10.1111/j.1365-2249.1991.tb05714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. J., Crisp S. J., Rose M. L., Taylor P. M., Yacoub M. H. Anti-endothelial antibodies and coronary artery disease after cardiac transplantation. Lancet. 1992 Jun 27;339(8809):1566–1570. doi: 10.1016/0140-6736(92)91832-s. [DOI] [PubMed] [Google Scholar]
- Habets W. J., de Rooij D. J., Salden M. H., Verhagen A. P., van Eekelen C. A., van de Putte L. B., van Venrooij W. J. Antibodies against distinct nuclear matrix proteins are characteristic for mixed connective tissue disease. Clin Exp Immunol. 1983 Oct;54(1):265–276. [PMC free article] [PubMed] [Google Scholar]
- Heurkens A. H., Gorter A., de Vreede T. M., Edgell C. S., Breedveld F. C., Daha M. R. Methods for the detection of anti-endothelial antibodies by enzyme-linked immunosorbent assay. J Immunol Methods. 1991 Jul 26;141(1):33–39. doi: 10.1016/0022-1759(91)90207-v. [DOI] [PubMed] [Google Scholar]
- Heurkens A. H., Hiemstra P. S., Lafeber G. J., Daha M. R., Breedveld F. C. Anti-endothelial cell antibodies in patients with rheumatoid arthritis complicated by vasculitis. Clin Exp Immunol. 1989 Oct;78(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland L. W., Gay R. E., Gay S. Collagen autoantibodies in patients with vasculitis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1991 Sep;60(3):412–418. doi: 10.1016/0090-1229(91)90097-t. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J., Pottinger B. E., Woo P., Black C. M., Loizou S., Byron M. A., Pearson J. D. Measurement and characterisation of circulating anti-endothelial cell IgG in connective tissue diseases. Clin Exp Immunol. 1988 Jun;72(3):450–456. [PMC free article] [PubMed] [Google Scholar]
- Savage C. O., Pottinger B. E., Gaskin G., Lockwood C. M., Pusey C. D., Pearson J. D. Vascular damage in Wegener's granulomatosis and microscopic polyarteritis: presence of anti-endothelial cell antibodies and their relation to anti-neutrophil cytoplasm antibodies. Clin Exp Immunol. 1991 Jul;85(1):14–19. doi: 10.1111/j.1365-2249.1991.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Termaat R. M., Brinkman K., van Gompel F., van den Heuvel L. P., Veerkamp J. H., Smeenk R. J., Berden J. H. Cross-reactivity of monoclonal anti-DNA antibodies with heparan sulfate is mediated via bound DNA/histone complexes. J Autoimmun. 1990 Oct;3(5):531–545. doi: 10.1016/s0896-8411(05)80019-8. [DOI] [PubMed] [Google Scholar]
- Westphal J. R., Willems H. W., Schalkwijk C. J., Ruiter D. J., de Waal R. M. A new 180-kDa dermal endothelial cell activation antigen: in vitro and in situ characteristics. J Invest Dermatol. 1993 Jan;100(1):27–34. doi: 10.1111/1523-1747.ep12349946. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]
- van der Zee J. M., Heurkens A. H., van der Voort E. A., Daha M. R., Breedveld F. C. Characterization of anti-endothelial antibodies in patients with rheumatoid arthritis complicated by vasculitis. Clin Exp Rheumatol. 1991 Nov-Dec;9(6):589–594. [PubMed] [Google Scholar]
- van der Zee J. M., Siegert C. E., de Vreede T. A., Daha M. R., Breedveld F. C. Characterization of anti-endothelial cell antibodies in systemic lupus erythematosus (SLE). Clin Exp Immunol. 1991 May;84(2):238–244. doi: 10.1111/j.1365-2249.1991.tb08155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



