Abstract
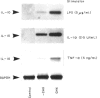
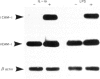
The role of mucosal fibroblasts in intestinal inflammatory reactions is not known. In this study, we demonstrate that fibroblasts grown from histologically normal human duodenal biopsy tissues expressed mRNA genes for granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) when stimulated with lipopolysaccharide (LPS) or IL-1 alpha. The increased mRNA expression of GM-CSF, IL-1 alpha, IL-1 beta, IL-6 and IL-8 in response to IL-1 alpha and LPS stimulation was time- and dose-dependent. In contrast, IL-10 was weakly expressed when fibroblasts were stimulated with LPS, IL-1 alpha or tumour necrosis factor-alpha (TNF-alpha), but the expression was enhanced in the presence of cycloheximide combined with optimal concentrations of LPS, IL-1 alpha or TNF-alpha, IL-1 alpha was a more potent stimulator than LPS for GM-CSF, IL-6, IL-8 and IL-10 expression, but not for IL-1 alpha and IL-1 beta. Increased GM-CSF, IL-6 and IL-8 gene expression was associated with the production of cytokine proteins in culture supernatant, but IL-1 alpha and IL-1 beta remained undetectable. Dexamethasone suppressed both gene expression and protein production of GM-CSF, IL-6 and IL-8 when fibroblasts were exposed to IL-1 alpha. TNF-alpha stimulated the release of GM-CSF, IL-6 and IL-8 and, combined with IL-1 alpha, cytokine production was enhanced synergistically. Finally, both LPS and IL-1 alpha up-regulated ICAM-1 and VCAM-1 gene expression. These findings implicate duodenal fibroblasts in the initiation and/or regulation of intestinal inflammation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akashi M., Loussararian A. H., Adelman D. C., Saito M., Koeffler H. P. Role of lymphotoxin in expression of interleukin 6 in human fibroblasts. Stimulation and regulation. J Clin Invest. 1990 Jan;85(1):121–129. doi: 10.1172/JCI114401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall H. H., Lane C., Ramser M. N., Anderson D. C. Induction of VCAM-1 and ICAM-1 on human neural cells and mechanisms of mononuclear leukocyte adherence. J Immunol. 1992 May 1;148(9):2717–2723. [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L., Lorre K., Van Damme J., Billiau A. Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. J Immunol. 1988 Dec 1;141(11):3868–3874. [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Elias J. A., Freundlich B., Kern J. A., Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990 Jun;97(6):1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabilize IL-6 messenger RNA. J Immunol. 1990 Jul 1;145(1):161–166. [PubMed] [Google Scholar]
- Fibbe W. E., Van Damme J., Billiau A., Duinkerken N., Lurvink E., Ralph P., Altrock B. W., Kaushansky K., Willemze R., Falkenburg J. H. Human fibroblasts produce granulocyte-CSF, macrophage-CSF, and granulocyte-macrophage-CSF following stimulation by interleukin-1 and poly(rI).poly(rC). Blood. 1988 Sep;72(3):860–866. [PubMed] [Google Scholar]
- Hamilton J. A., Waring P. M., Filonzi E. L. Induction of leukemia inhibitory factor in human synovial fibroblasts by IL-1 and tumor necrosis factor-alpha. J Immunol. 1993 Feb 15;150(4):1496–1502. [PubMed] [Google Scholar]
- Helfgott D. C., May L. T., Sthoeger Z., Tamm I., Sehgal P. B. Bacterial lipopolysaccharide (endotoxin) enhances expression and secretion of beta 2 interferon by human fibroblasts. J Exp Med. 1987 Nov 1;166(5):1300–1309. doi: 10.1084/jem.166.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Taga T., Nakano N., Yasukawa K., Kashiwamura S., Shimizu K., Nakajima K., Pyun K. H., Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A. 1985 Aug;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvale D., Krajci P., Brandtzaeg P. Expression and regulation of adhesion molecules ICAM-1 (CD54) and LFA-3 (CD58) in human intestinal epithelial cell lines. Scand J Immunol. 1992 Jun;35(6):669–676. doi: 10.1111/j.1365-3083.1992.tb02973.x. [DOI] [PubMed] [Google Scholar]
- Le J. M., Weinstein D., Gubler U., Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J Immunol. 1987 Apr 1;138(7):2137–2142. [PubMed] [Google Scholar]
- Leizer T., Cebon J., Layton J. E., Hamilton J. A. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. Induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990 Nov 15;76(10):1989–1996. [PubMed] [Google Scholar]
- Lotz M., Guerne P. A. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA). J Biol Chem. 1991 Feb 5;266(4):2017–2020. [PubMed] [Google Scholar]
- Malizia G., Calabrese A., Cottone M., Raimondo M., Trejdosiewicz L. K., Smart C. J., Oliva L., Pagliaro L. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology. 1991 Jan;100(1):150–159. doi: 10.1016/0016-5085(91)90595-c. [DOI] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Pang G., Buret A., Batey R. T., Chen Q. Y., Couch L., Cripps A., Clancy R. Morphological, phenotypic and functional characteristics of a pure population of CD56+ CD16- CD3- large granular lymphocytes generated from human duodenal mucosa. Immunology. 1993 Jul;79(3):498–505. [PMC free article] [PubMed] [Google Scholar]
- Phipps R. P., Penney D. P., Keng P., Silvera M., Harkins S., Derdak S. Immune functions of subpopulations of lung fibroblasts. Immunol Res. 1990;9(4):275–286. doi: 10.1007/BF02935527. [DOI] [PubMed] [Google Scholar]
- Pullman W. E., Elsbury S., Kobayashi M., Hapel A. J., Doe W. F. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992 Feb;102(2):529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- Rolfe M. W., Kunkel S. L., Standiford T. J., Chensue S. W., Allen R. M., Evanoff H. L., Phan S. H., Strieter R. M. Pulmonary fibroblast expression of interleukin-8: a model for alveolar macrophage-derived cytokine networking. Am J Respir Cell Mol Biol. 1991 Nov;5(5):493–501. doi: 10.1165/ajrcmb/5.5.493. [DOI] [PubMed] [Google Scholar]
- Scott S., Pandolfi F., Kurnick J. T. Fibroblasts mediate T cell survival: a proposed mechanism for retention of primed T cells. J Exp Med. 1990 Dec 1;172(6):1873–1876. doi: 10.1084/jem.172.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart C. J., Calabrese A., Oakes D. J., Howdle P. D., Trejdosiewicz L. K. Expression of the LFA-1 beta 2 integrin (CD11a/CD18) and ICAM-1 (CD54) in normal and coeliac small bowel mucosa. Scand J Immunol. 1991 Sep;34(3):299–305. doi: 10.1111/j.1365-3083.1991.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Stevens C., Walz G., Singaram C., Lipman M. L., Zanker B., Muggia A., Antonioli D., Peppercorn M. A., Strom T. B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992 Jun;37(6):818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- Sturgess R. P., Macartney J. C., Makgoba M. W., Hung C. H., Haskard D. O., Ciclitira P. J. Differential upregulation of intercellular adhesion molecule-1 in coeliac disease. Clin Exp Immunol. 1990 Dec;82(3):489–492. doi: 10.1111/j.1365-2249.1990.tb05477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga K., Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992 Feb 15;148(4):1143–1148. [PubMed] [Google Scholar]
- Thompson-Snipes L., Dhar V., Bond M. W., Mosmann T. R., Moore K. W., Rennick D. M. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991 Feb 1;173(2):507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Schaafsma M. R., Fibbe W. E., Falkenburg J. H., Opdenakker G., Billiau A. Simultaneous production of interleukin 6, interferon-beta and colony-stimulating activity by fibroblasts after viral and bacterial infection. Eur J Immunol. 1989 Jan;19(1):163–168. doi: 10.1002/eji.1830190126. [DOI] [PubMed] [Google Scholar]
- Vancheri C., Ohtoshi T., Cox G., Xaubet A., Abrams J. S., Gauldie J., Dolovich J., Denburg J., Jordana M. Neutrophilic differentiation induced by human upper airway fibroblast-derived granulocyte/macrophage colony-stimulating factor (GM-CSF). Am J Respir Cell Mol Biol. 1991 Jan;4(1):11–17. doi: 10.1165/ajrcmb/4.1.11. [DOI] [PubMed] [Google Scholar]
- Wilkinson L. S., Edwards J. C., Poston R. N., Haskard D. O. Expression of vascular cell adhesion molecule-1 in normal and inflamed synovium. Lab Invest. 1993 Jan;68(1):82–88. [PubMed] [Google Scholar]
- Xing Z., Jordana M., Braciak T., Ohtoshi T., Gauldie J. Lipopolysaccharide induces expression of granulocyte/macrophage colony-stimulating factor, interleukin-8, and interleukin-6 in human nasal, but not lung, fibroblasts: evidence for heterogeneity within the respiratory tract. Am J Respir Cell Mol Biol. 1993 Sep;9(3):255–263. doi: 10.1165/ajrcmb/9.3.255. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]