Abstract
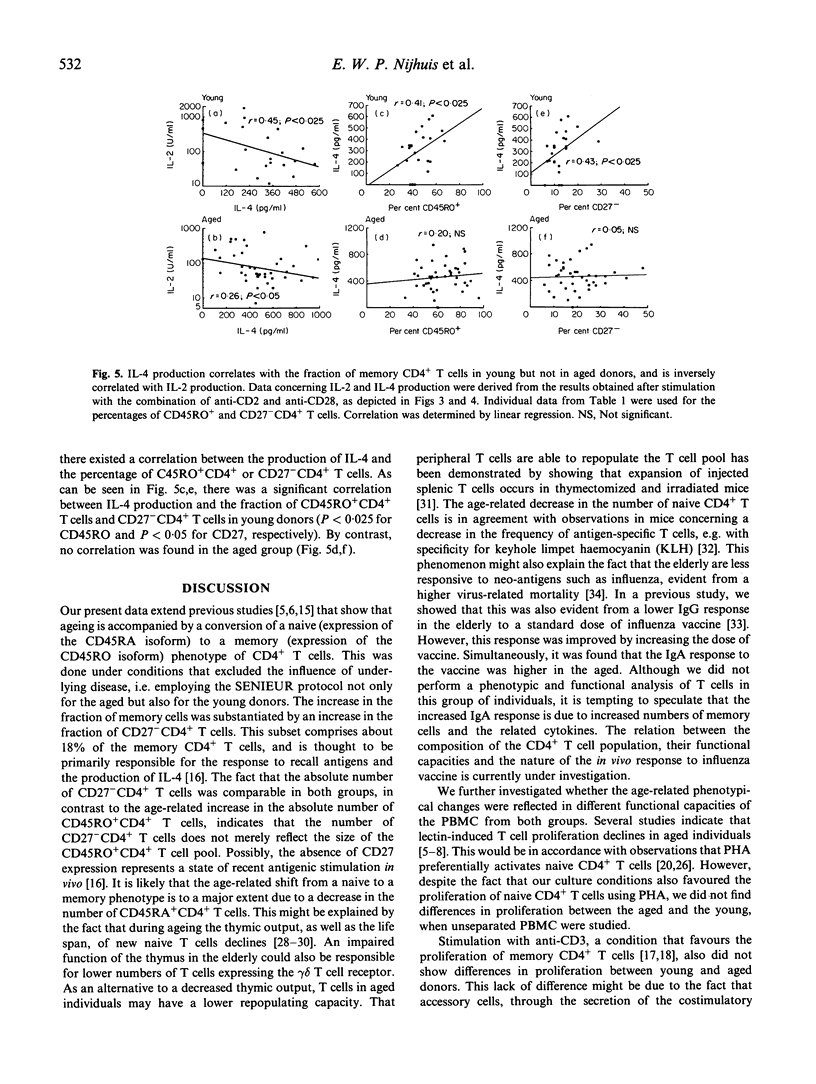
The influence of ageing on phenotype and function of CD4+ T cells was studied by comparing young (19-28 years of age) and aged (75-84 years of age) donors that were selected using the SENIEUR protocol to exclude underlying disease. An age-related increase was observed in the relative number of memory cells, not only on the basis of a decreased CD45RA and increased CD45RO expression, but also on the basis of a decrease in the fraction of CD27+CD4+ T cells. Our observation that the absolute number of CD45RO+CD4+ T cells was increased, while absolute numbers of CD27-CD4+ T cells remained unchanged in aged donors, indicates that the latter subset does not merely reflect the size of the CD45RO+CD4+ T cell pool. The increased fraction of memory cells in the aged was functionally reflected in an increased IL-4 production and T cell proliferation, when cells were activated with the combination of anti-CD2 and anti-CD28, whereas IL-2 production was comparable between both groups. No differences were observed with respect to proliferative T cell responses or IL-2 production using plate-bound anti-CD3 or phytohaemagglutinin (PHA). The observation that IL-4 production correlated with the fraction of memory cells in young donors but not in aged donors suggests different functional characteristics of this subset in aged donors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbee R. A., Halonen M., Lebowitz M., Burrows B. Distribution of IgE in a community population sample: correlations with age, sex, and allergen skin test reactivity. J Allergy Clin Immunol. 1981 Aug;68(2):106–111. doi: 10.1016/0091-6749(81)90167-6. [DOI] [PubMed] [Google Scholar]
- Beckman I., Dimopoulos K., Xu X. N., Ahern M., Bradley J. Age-related changes in the activation requirements of human CD4+ T-cell subsets. Cell Immunol. 1991 Jan;132(1):17–25. doi: 10.1016/0008-8749(91)90003-t. [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Duncan D. D., Yoshimoto K., Swain S. L. Memory effectors: a potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen. J Immunol. 1993 Apr 15;150(8 Pt 1):3119–3130. [PubMed] [Google Scholar]
- Byrne J. A., Butler J. L., Cooper M. D. Differential activation requirements for virgin and memory T cells. J Immunol. 1988 Nov 15;141(10):3249–3257. [PubMed] [Google Scholar]
- Chopra R. K., Holbrook N. J., Powers D. C., McCoy M. T., Adler W. H., Nagel J. E. Interleukin 2, interleukin 2 receptor, and interferon-gamma synthesis and mRNA expression in phorbol myristate acetate and calcium ionophore A23187-stimulated T cells from elderly humans. Clin Immunol Immunopathol. 1989 Nov;53(2 Pt 1):297–308. doi: 10.1016/0090-1229(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- De Jong R., Brouwer M., Hooibrink B., Van der Pouw-Kraan T., Miedema F., Van Lier R. A. The CD27- subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol. 1992 Apr;22(4):993–999. doi: 10.1002/eji.1830220418. [DOI] [PubMed] [Google Scholar]
- De Paoli P., Battistin S., Santini G. F. Age-related changes in human lymphocyte subsets: progressive reduction of the CD4 CD45R (suppressor inducer) population. Clin Immunol Immunopathol. 1988 Sep;48(3):290–296. doi: 10.1016/0090-1229(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Sjögren H. O., Carlsson R. Two subsets of human CD4+ T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-gamma can be defined by the Leu-18 and UCHL1 monoclonal antibodies. Eur J Immunol. 1988 Aug;18(8):1173–1178. doi: 10.1002/eji.1830180805. [DOI] [PubMed] [Google Scholar]
- Ernst D. N., Weigle W. O., McQuitty D. N., Rothermel A. L., Hobbs M. V. Stimulation of murine T cell subsets with anti-CD3 antibody. Age-related defects in the expression of early activation molecules. J Immunol. 1989 Mar 1;142(5):1413–1421. [PubMed] [Google Scholar]
- Felser J. M., Raff M. J. Infectious diseases and aging: immunologic perspectives. J Am Geriatr Soc. 1983 Dec;31(12):802–807. doi: 10.1111/j.1532-5415.1983.tb03403.x. [DOI] [PubMed] [Google Scholar]
- Gardner I. D. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980 Sep-Oct;2(5):801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Kozak R., Durante M., Weksler M. E. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981 Apr;67(4):937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann A., Ledbetter J. A., Rabinovitch P. S. Reduced proliferation in T lymphocytes in aged humans is predominantly in the CD8+ subset, and is unrelated to defects in transmembrane signaling which are predominantly in the CD4+ subset. Exp Cell Res. 1989 Feb;180(2):367–382. doi: 10.1016/0014-4827(89)90064-5. [DOI] [PubMed] [Google Scholar]
- Hirokawa K., Utsuyama M., Katsura Y., Sado T. Influence of age on the proliferation and peripheralization of thymic T cells. Arch Pathol Lab Med. 1988 Jan;112(1):13–21. [PubMed] [Google Scholar]
- Horgan K. J., Van Seventer G. A., Shimizu Y., Shaw S. Hyporesponsiveness of "naive" (CD45RA+) human T cells to multiple receptor-mediated stimuli but augmentation of responses by co-stimuli. Eur J Immunol. 1990 May;20(5):1111–1118. doi: 10.1002/eji.1830200525. [DOI] [PubMed] [Google Scholar]
- Ligthart G. J., Corberand J. X., Geertzen H. G., Meinders A. E., Knook D. L., Hijmans W. Necessity of the assessment of health status in human immunogerontological studies: evaluation of the SENIEUR protocol. Mech Ageing Dev. 1990 Jul;55(1):89–105. doi: 10.1016/0047-6374(90)90108-r. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Anderson P., Daley J. F., Schlossman S., Morimoto C. CD4+CD45R+ cells are preferentially activated through the CD2 pathway. Eur J Immunol. 1988 Sep;18(9):1473–1476. doi: 10.1002/eji.1830180926. [DOI] [PubMed] [Google Scholar]
- Miller R. A. Age-associated decline in precursor frequency for different T cell-mediated reactions, with preservation of helper or cytotoxic effect per precursor cell. J Immunol. 1984 Jan;132(1):63–68. [PubMed] [Google Scholar]
- Miller R. A., Stutman O. T cell repopulation from functionally restricted splenic progenitors: 10,000-fold expansion documented by using limiting dilution analyses. J Immunol. 1984 Dec;133(6):2925–2932. [PubMed] [Google Scholar]
- Murasko D. M., Weiner P., Kaye D. Decline in mitogen induced proliferation of lymphocytes with increasing age. Clin Exp Immunol. 1987 Nov;70(2):440–448. [PMC free article] [PubMed] [Google Scholar]
- Nagelkerken L., Hertogh-Huijbregts A., Dobber R., Dräger A. Age-related changes in lymphokine production related to a decreased number of CD45RBhi CD4+ T cells. Eur J Immunol. 1991 Feb;21(2):273–281. doi: 10.1002/eji.1830210206. [DOI] [PubMed] [Google Scholar]
- Nagelkerken L., Hertogh-Huijbregts A. The acquisition of a memory phenotype by murine CD4+ T cells is accompanied by a loss in their capacity to increase intracellular calcium. Dev Immunol. 1992;3(1):25–34. doi: 10.1155/1992/31573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhuis E. W., Nagelkerken L. Age-related changes in immune reactivity: the influence of intrinsic defects and of a changed composition of the CD4+ T cell compartment. Exp Clin Immunogenet. 1992;9(4):195–202. [PubMed] [Google Scholar]
- Noorloos A. A., van Beek A. A., Melief C. J. Cryopreservation of cells for immunological typing of non-Hodgkin's lymphomas. Cancer Res. 1980 Aug;40(8 Pt 1):2890–2894. [PubMed] [Google Scholar]
- O'Leary J. J., Hallgren H. M. Aging and lymphocyte function: a model for testing gerontologic hypotheses of aging in man. Arch Gerontol Geriatr. 1991 Mar-Jun;12(2-3):199–218. doi: 10.1016/0167-4943(91)90028-o. [DOI] [PubMed] [Google Scholar]
- Remarque E. J., van Beek W. C., Ligthart G. J., Borst R. J., Nagelkerken L., Palache A. M., Sprenger M. J., Masurel N. Improvement of the immunoglobulin subclass response to influenza vaccine in elderly nursing-home residents by the use of high-dose vaccines. Vaccine. 1993;11(6):649–654. doi: 10.1016/0264-410x(93)90311-k. [DOI] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Bacon P. A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from human peripheral blood and by human CD4+ T cell clones. J Immunol. 1989 Aug 1;143(3):907–912. [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Gaston J. S., Bacon P. A. Interleukin-2 production and response by helper T-cell subsets in man. Immunology. 1988 Sep;65(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., June C. H., Young H. A., Shaw S. Enhanced responsiveness of human memory T cells to CD2 and CD3 receptor-mediated activation. Eur J Immunol. 1989 May;19(5):803–808. doi: 10.1002/eji.1830190504. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Schneider E. L. Infectious diseases in the elderly. Ann Intern Med. 1983 Mar;98(3):395–400. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- Schuurman H. J., Nagelkerken L., De Weger R. A., Rozing J. Age-associated involution: significance of a physiologic process. Thymus. 1991 Aug;18(1):1–6. [PubMed] [Google Scholar]
- Scollay R. G., Butcher E. C., Weissman I. L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980 Mar;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. The cellular and subcellular bases of immunosenescence. Adv Immunol. 1989;46:221–261. doi: 10.1016/s0065-2776(08)60655-0. [DOI] [PubMed] [Google Scholar]
- Van der Pouw-Kraan T., Van Kooten C., Rensink I., Aarden L. Interleukin (IL)-4 production by human T cells: differential regulation of IL-4 vs. IL-2 production. Eur J Immunol. 1992 May;22(5):1237–1241. doi: 10.1002/eji.1830220519. [DOI] [PubMed] [Google Scholar]
- van Lier R. A., Brouwer M., Rebel V. I., van Noesel C. J., Aarden L. A. Immobilized anti-CD3 monoclonal antibodies induce accessory cell-independent lymphokine production, proliferation and helper activity in human T lymphocytes. Immunology. 1989 Sep;68(1):45–50. [PMC free article] [PubMed] [Google Scholar]



