Abstract
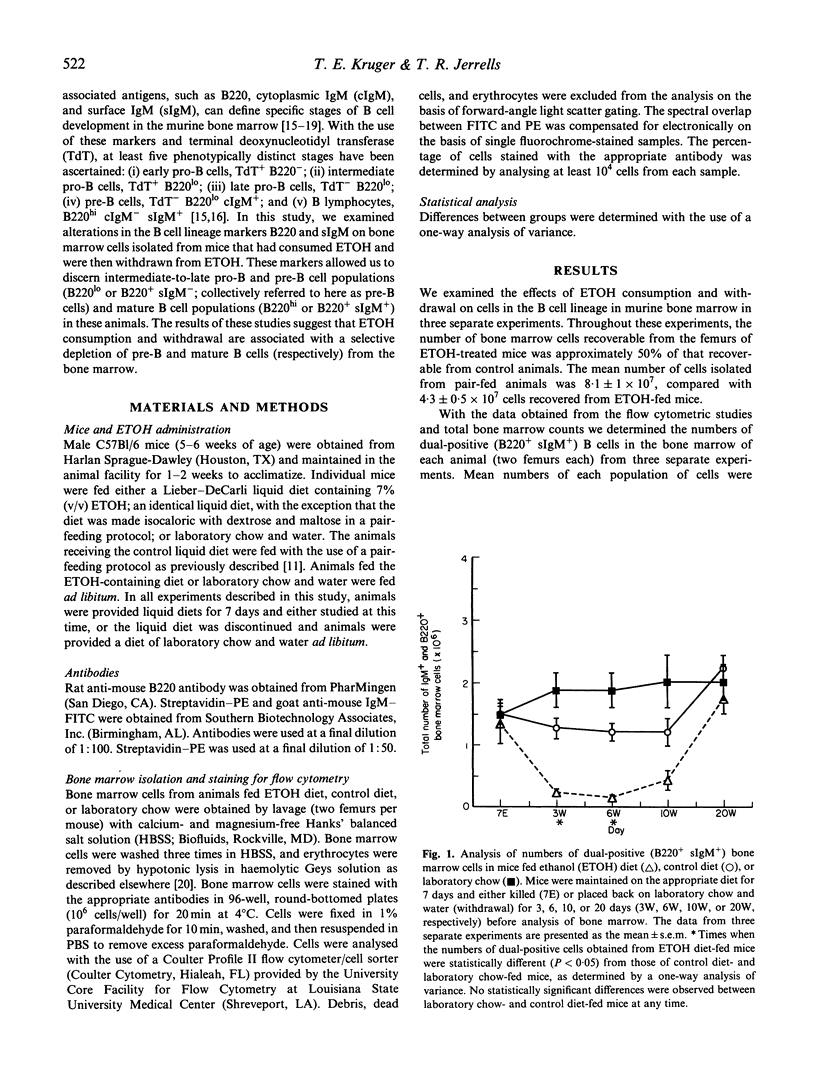
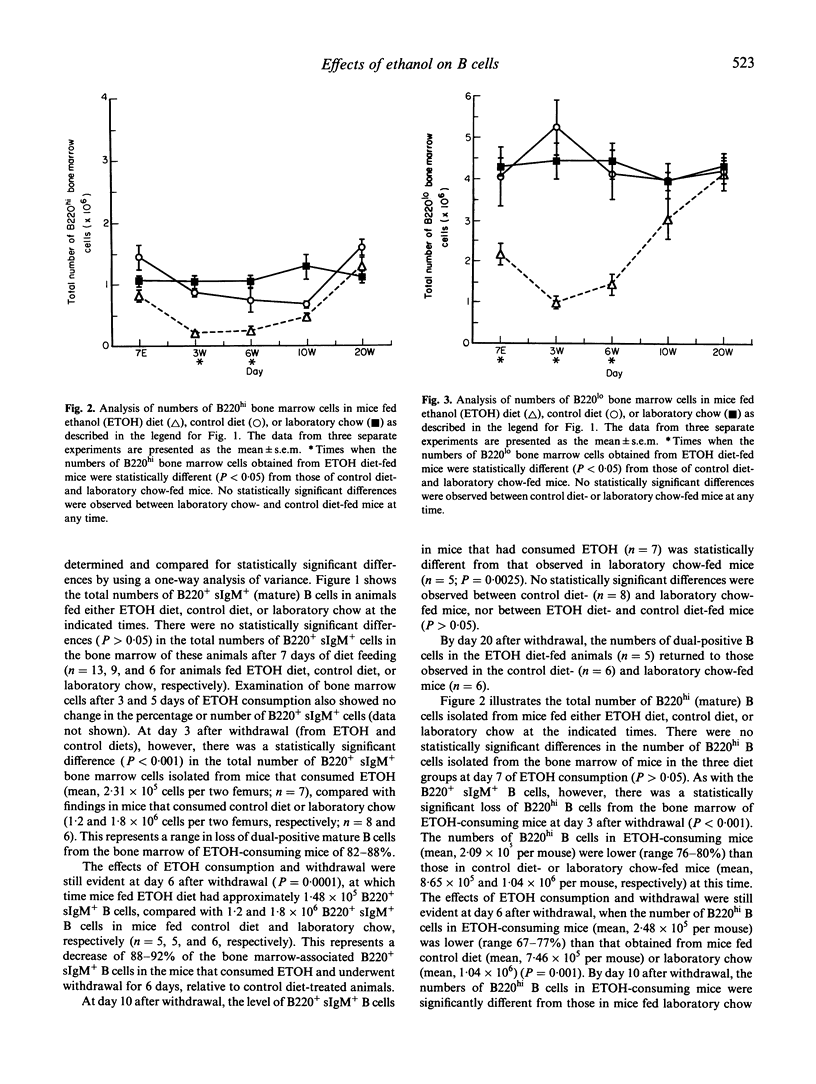
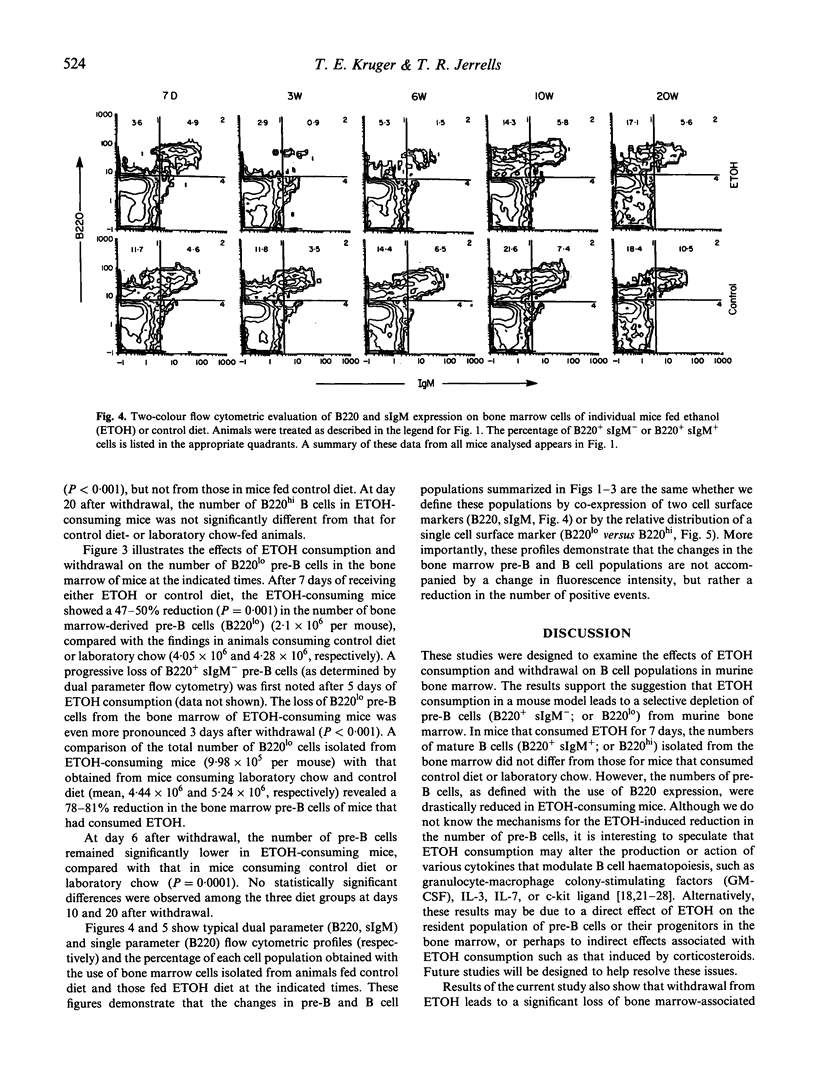
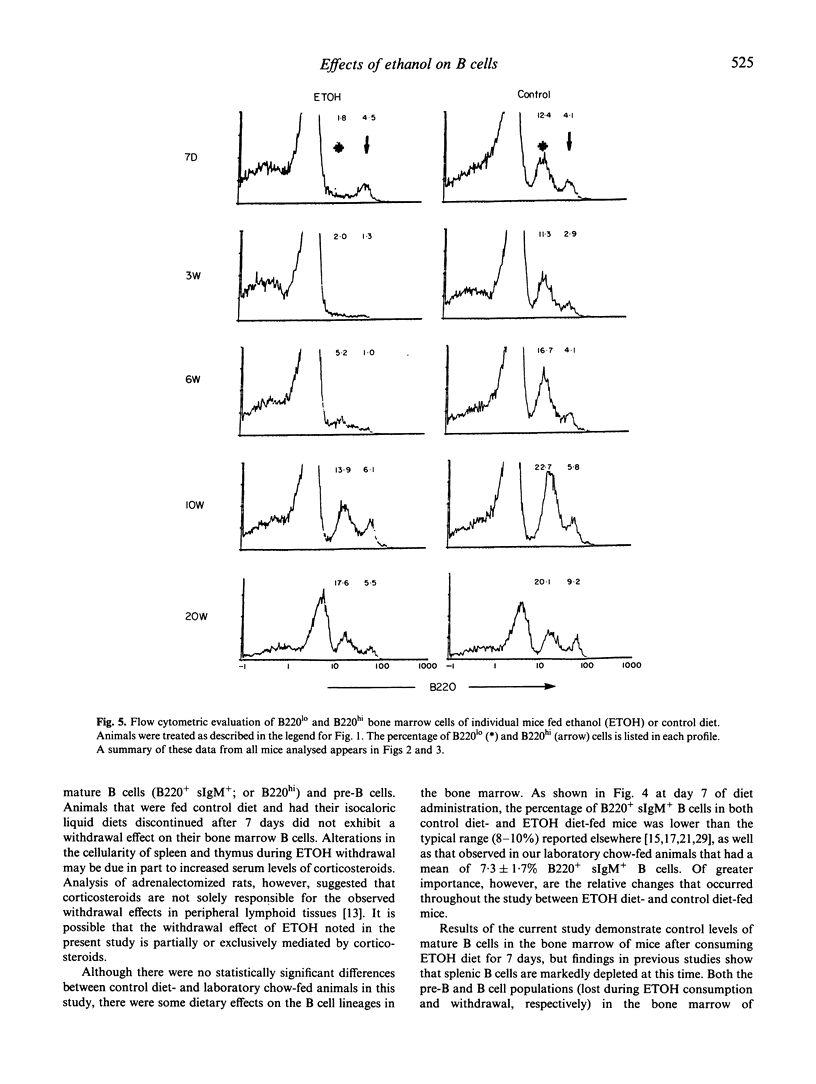
We designed studies to examine the effects of ethanol consumption and withdrawal on the numbers of pre-B and B cells in murine bone marrow. Flow cytometric analysis of B220 and surface IgM expression on bone marrow cells revealed that consumption of ethanol by mice for 7 days led to a significant reduction in pre-B cells. The number of mature B cells in the bone marrow of these animals, however, did not differ from that of control mice. In contrast, examination of bone marrow obtained from mice at various times after withdrawal from ethanol showed significantly fewer numbers of mature B cells and an even greater loss of pre-B cells. This effect was seen for relatively long periods after withdrawal. These study findings are interpreted to suggest that ethanol consumption results in changes in the pre-B cell population in murine bone marrow. It also appears that withdrawal from ethanol results in more profound changes in the mature B cell population of the bone marrow than those that occur during ethanol consumption.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Lyman S. D., Baird A., Wignall J. M., Eisenman J., Rauch C., March C. J., Boswell H. S., Gimpel S. D., Cosman D. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990 Oct 5;63(1):235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Howeedy A., Kajdacsy-Balla A. Macrophage function in chronic experimental alcoholism. I. Modulation of surface receptors and phagocytosis. Immunology. 1988 Nov;65(3):405–409. [PMC free article] [PubMed] [Google Scholar]
- Breeden J. H. Alcohol, alcoholism, and cancer. Med Clin North Am. 1984 Jan;68(1):163–177. doi: 10.1016/s0025-7125(16)31248-2. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. In vivo administration of recombinant granulocyte-macrophage colony-stimulating factor results in a reversible inhibition of primary B lymphopoiesis. J Immunol. 1991 Jun 15;146(12):4204–4208. [PubMed] [Google Scholar]
- Fulop G. M., Osmond D. G. Regulation of bone marrow lymphocyte production. III. Increased production of B and non-B lymphocytes after administering systemic antigens. Cell Immunol. 1983 Jan;75(1):80–90. doi: 10.1016/0008-8749(83)90307-6. [DOI] [PubMed] [Google Scholar]
- Fulop G. M., Osmond D. G. Regulation of bone marrow lymphocyte production. IV. Cells mediating the stimulation of marrow lymphocyte production by sheep red blood cells: studies in anti-IgM-suppressed mice, athymic mice, and silica-treated mice. Cell Immunol. 1983 Jan;75(1):91–102. doi: 10.1016/0008-8749(83)90308-8. [DOI] [PubMed] [Google Scholar]
- Förster I., Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhus N. E., Matre R. In vitro effect of ethanol on subpopulations of human blood mononuclear cells. Int Arch Allergy Appl Immunol. 1982;68(4):382–386. doi: 10.1159/000233130. [DOI] [PubMed] [Google Scholar]
- Gluckman S. J., Dvorak V. C., MacGregor R. R. Host defenses during prolonged alcohol consumption in a controlled environment. Arch Intern Med. 1977 Nov;137(11):1539–1543. [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Kunisada T., Ogawa M., Sudo T., Kodama H., Suda T., Nishikawa S., Nishikawa S. Stepwise progression of B lineage differentiation supported by interleukin 7 and other stromal cell molecules. J Exp Med. 1990 May 1;171(5):1683–1695. doi: 10.1084/jem.171.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S. Interleukin 7: effects on early events in lymphopoiesis. Immunol Today. 1989 May;10(5):170–173. doi: 10.1016/0167-5699(89)90175-8. [DOI] [PubMed] [Google Scholar]
- Huang E., Nocka K., Beier D. R., Chu T. Y., Buck J., Lahm H. W., Wellner D., Leder P., Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990 Oct 5;63(1):225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Marietta C. A., Weight F. F., Eckardt M. J. Effect of adrenalectomy on ethanol-associated immunosuppression. Int J Immunopharmacol. 1990;12(4):435–442. doi: 10.1016/0192-0561(90)90027-k. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Smith W., Eckardt M. J. Murine model of ethanol-induced immunosuppression. Alcohol Clin Exp Res. 1990 Aug;14(4):546–550. doi: 10.1111/j.1530-0277.1990.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H., Kincade P. W., Good R. A. Immunosuppression of marrow B lymphocytes by administration of Corynebacterium parvum in mice. J Immunol. 1981 Dec;127(6):2502–2507. [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Paige C. J., Scheid M. P. Cellular interactions affecting the maturation of murine B lymphocyte precursors in vitro. J Immunol. 1981 Jul;127(1):255–260. [PubMed] [Google Scholar]
- MacGregor R. R. Alcohol and immune defense. JAMA. 1986 Sep 19;256(11):1474–1479. [PubMed] [Google Scholar]
- Martin F. H., Suggs S. V., Langley K. E., Lu H. S., Ting J., Okino K. H., Morris C. F., McNiece I. K., Jacobsen F. W., Mendiaz E. A. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990 Oct 5;63(1):203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- McCarthy S. P., Lewis C. E., McGee J. O. Effects of ethanol on human monocyte/macrophage lysozyme storage and release. Implications for the pathobiology of alcoholic liver disease. J Hepatol. 1990 Jan;10(1):90–98. doi: 10.1016/0168-8278(90)90078-6. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Conlon P., Charrier K., Braddy S., Alpert A., Williams D., Namen A. E., Mochizuki D. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J Immunol. 1991 Jul 15;147(2):561–568. [PubMed] [Google Scholar]
- Mutchnick M. G., Missirian A., Johnson A. G. Lymphocyte cytotoxicity in human liver disease using rat hepatocyte monolayer cultures. Clin Immunol Immunopathol. 1980 Aug;16(4):423–437. doi: 10.1016/0090-1229(80)90184-1. [DOI] [PubMed] [Google Scholar]
- Mørland B., Mørland J. Effects of long-term ethanol consumption on rat peritoneal macrophages. Acta Pharmacol Toxicol (Copenh) 1982 Mar;50(3):221–224. doi: 10.1111/j.1600-0773.1982.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Osmond D. G. Proliferation kinetics and the lifespan of B cells in central and peripheral lymphoid organs. Curr Opin Immunol. 1991 Apr;3(2):179–185. doi: 10.1016/0952-7915(91)90047-5. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Osmond D. G. Regulation of early precursor B cell proliferation in mouse bone marrow: stimulation by exogenous agents mediated by macrophages in the spleen. Cell Immunol. 1991 Jun;135(1):168–183. doi: 10.1016/0008-8749(91)90263-b. [DOI] [PubMed] [Google Scholar]
- Pietrangeli C. E., Osmond D. G. Regulation of B-lymphocyte production in the bone marrow: mediation of the effects of exogenous stimulants by adoptively transferred spleen cells. Cell Immunol. 1987 Jul;107(2):348–357. doi: 10.1016/0008-8749(87)90243-7. [DOI] [PubMed] [Google Scholar]
- Pietrangeli C. E., Osmond D. G. Regulation of B-lymphocyte production in the bone marrow: role of macrophages and the spleen in mediating responses to exogenous agents. Cell Immunol. 1985 Aug;94(1):147–158. doi: 10.1016/0008-8749(85)90092-9. [DOI] [PubMed] [Google Scholar]
- Saad A. J., Domiati-Saad R., Jerrells T. R. Ethanol ingestion increases susceptibility of mice to Listeria monocytogenes. Alcohol Clin Exp Res. 1993 Feb;17(1):75–85. doi: 10.1111/j.1530-0277.1993.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Saad A. J., Jerrells T. R. Flow cytometric and immunohistochemical evaluation of ethanol-induced changes in splenic and thymic lymphoid cell populations. Alcohol Clin Exp Res. 1991 Oct;15(5):796–803. doi: 10.1111/j.1530-0277.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Smith W. I., Jr, Van Thiel D. H., Whiteside T., Janoson B., Magovern J., Puet T., Rabin B. S. Altered immunity in male patients with alcoholic liver disease: evidence for defective immune regulation. Alcohol Clin Exp Res. 1980 Apr;4(2):199–206. doi: 10.1111/j.1530-0277.1980.tb05635.x. [DOI] [PubMed] [Google Scholar]
- Sudo T., Ito M., Ogawa Y., Iizuka M., Kodama H., Kunisada T., Hayashi S., Ogawa M., Sakai K., Nishikawa S. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989 Jul 1;170(1):333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundblad A., Marcos M., Huetz F., Freitas A., Heusser C., Portnoï D., Coutinho A. Normal serum immunoglobulins influence the numbers of bone marrow pre-B and B cells. Eur J Immunol. 1991 May;21(5):1155–1161. doi: 10.1002/eji.1830210510. [DOI] [PubMed] [Google Scholar]
- Young G. P., Van der Weyden M. B., Rose I. S., Dudley F. J. Lymphopenia and lymphocyte transformation in alcoholics. Experientia. 1979 Feb 15;35(2):268–269. doi: 10.1007/BF01920656. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]



