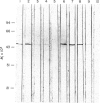
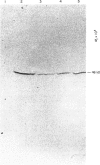
Abstract
Chronic hepatitis delta virus infection is associated with the presence of autoantibodies to rat forestomach and thymus in approximately 60% of patients' sera. We have characterized the antigen against which these autoantibodies are directed as a protein of 46 kD by immunoblotting studies on rat forestomach and thymus extracts. Normal human sera or sera from patients with other hepatic or non-hepatic autoimmune disorders did not bind to this protein. The immunoblot assay was more sensitive than immunofluorescence. Maximal titre was 1:10,000 versus 1:5120. By techniques of elution of specific antibodies from immunoblots, our results showed that the same antigen was present in both tissues. This antigen did not share common epitopes with hepatitis delta virus (HDV). Patients' sera depleted of basal cell layer and thymic stellate epithelial cell antibodies by absorption with the corresponding tissue extract maintained the HDV antibody titres. The autoimmune phenomena observed in patients with HDV infection seems to be a colateral process induced by the replication of delta virus in the host.
Full text
PDF
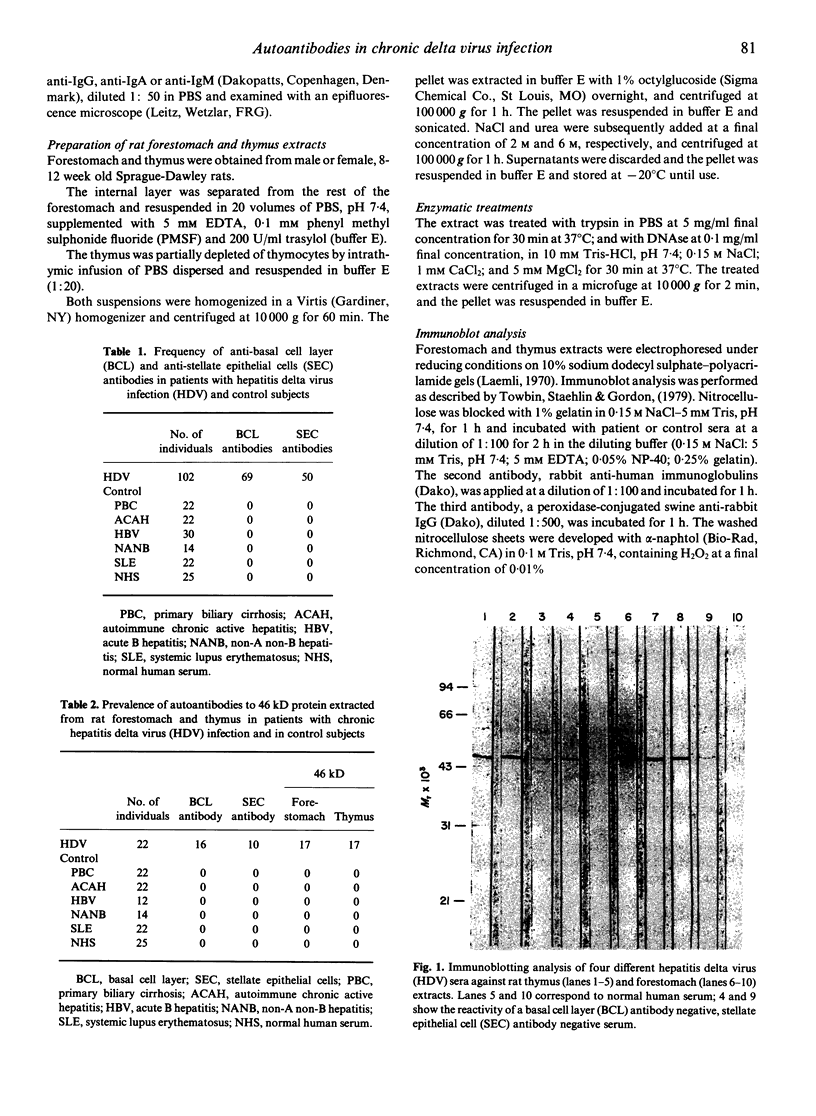
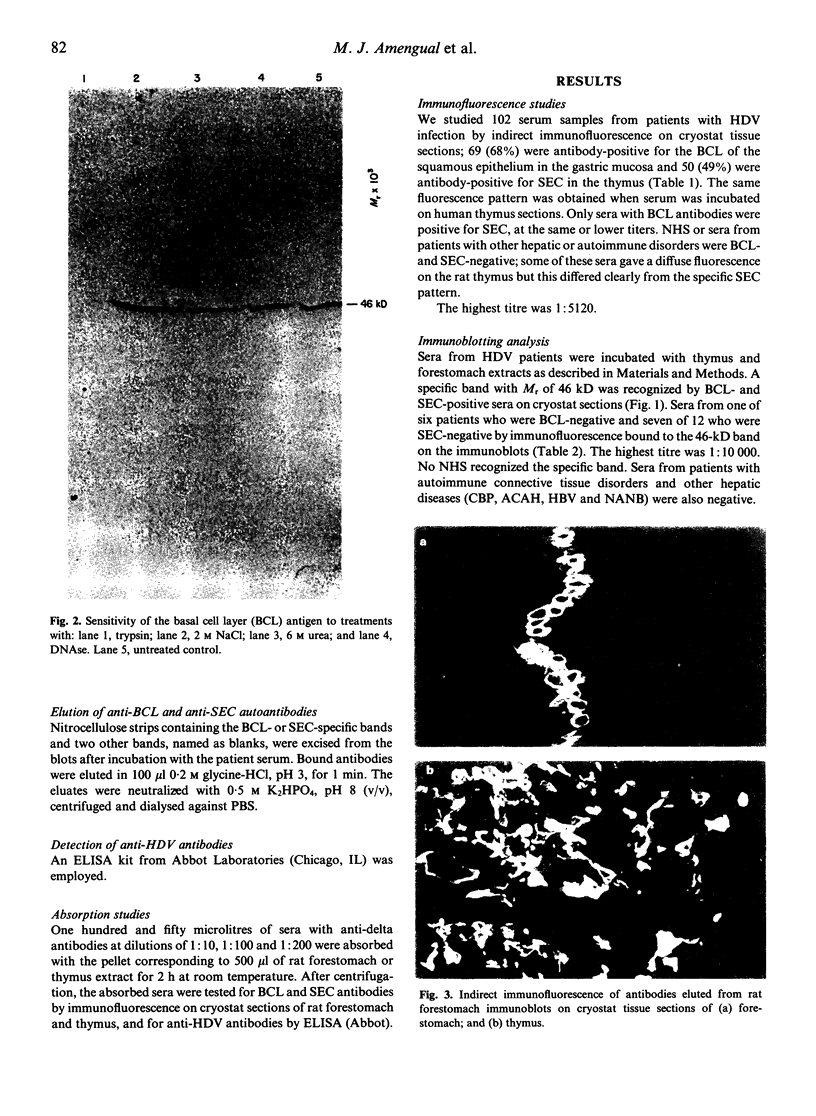


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann K. F., Gerin J. L. Antigens of hepatitis delta virus in the liver and serum of humans and animals. J Infect Dis. 1986 Oct;154(4):702–706. doi: 10.1093/infdis/154.4.702. [DOI] [PubMed] [Google Scholar]
- Bonino F., Heermann K. H., Rizzetto M., Gerlich W. H. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986 Jun;58(3):945–950. doi: 10.1128/jvi.58.3.945-950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivelli O., Lavarini C., Chiaberge E., Amoroso A., Farci P., Negro F., Rizzetto M. Microsomal autoantibodies in chronic infection with the HBsAg associated delta (delta) agent. Clin Exp Immunol. 1983 Oct;54(1):232–238. [PMC free article] [PubMed] [Google Scholar]
- Hirokawa K., McClure J. E., Goldstein A. L. Age-related changes in localization of thymosin in the human thymus. Thymus. 1982 Jan;4(1):19–29. [PubMed] [Google Scholar]
- Homberg J. C., Abuaf N., Bernard O., Islam S., Alvarez F., Khalil S. H., Poupon R., Darnis F., Lévy V. G., Grippon P. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of "autoimmune" hepatitis. Hepatology. 1987 Nov-Dec;7(6):1333–1339. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- Homberg J. C., Andre C., Abuaf N. A new anti-liver-kidney microsome antibody (anti-LKM2) in tienilic acid-induced hepatitis. Clin Exp Immunol. 1984 Mar;55(3):561–570. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenkei R., Biberfeld G., Buligescu L., Tovaru S., Biberfeld P., Hilborn L., Magnius L. Autoantibodies against the basal cell layer: an association with chronic hepatitis B. Clin Immunol Immunopathol. 1983 Mar;26(3):436–441. doi: 10.1016/0090-1229(83)90129-0. [DOI] [PubMed] [Google Scholar]
- Lenkei R., Biberfeld G., Magnius L. O., Fagraeus A., Biberfeld P. Autoantibodies to the basal cells of squamous epithelium react with thymic epithelial cells. Clin Immunol Immunopathol. 1985 Jan;34(1):11–19. doi: 10.1016/0090-1229(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Magnius L. O., Lenkei R., Norder H., Biberfeld G., Mushahwar I. K. Autoantibodies to thymic epithelial cells in hepatitis B virus-associated delta infection. J Infect Dis. 1985 Jul;152(1):232–232. doi: 10.1093/infdis/152.1.232. [DOI] [PubMed] [Google Scholar]
- McFarland E. J., Scearce R. M., Haynes B. F. The human thymic microenvironment: cortical thymic epithelium is an antigenically distinct region of the thymic microenvironment. J Immunol. 1984 Sep;133(3):1241–1249. [PubMed] [Google Scholar]
- Pohl C., Baroudy B. M., Bergmann K. F., Cote P. J., Purcell R. H., Hoofnagle J., Gerin J. L. A human monoclonal antibody that recognizes viral polypeptides and in vitro translation products of the genome of the hepatitis D virus. J Infect Dis. 1987 Oct;156(4):622–629. doi: 10.1093/infdis/156.4.622. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Swana G., Doniach D. Microsomal antibodies in active chronic hepatitis and other disorders. Clin Exp Immunol. 1973 Nov;15(3):331–344. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli D., Crespi C., Bianchi F. B., Pisi E. Immunofluorescent detection of anti-cytoskeleton antibodies using vinblastine-treated mononuclear cells. J Immunol Methods. 1985 Sep 3;82(1):77–82. doi: 10.1016/0022-1759(85)90226-1. [DOI] [PubMed] [Google Scholar]
- Zauli D., Fusconi M., Crespi C., Bianchi F. B., Craxi A., Pisi E. Close association between basal cell layer antibodies and hepatitis B virus-associated chronic delta infection. Hepatology. 1984 Nov-Dec;4(6):1103–1106. doi: 10.1002/hep.1840040601. [DOI] [PubMed] [Google Scholar]