Abstract
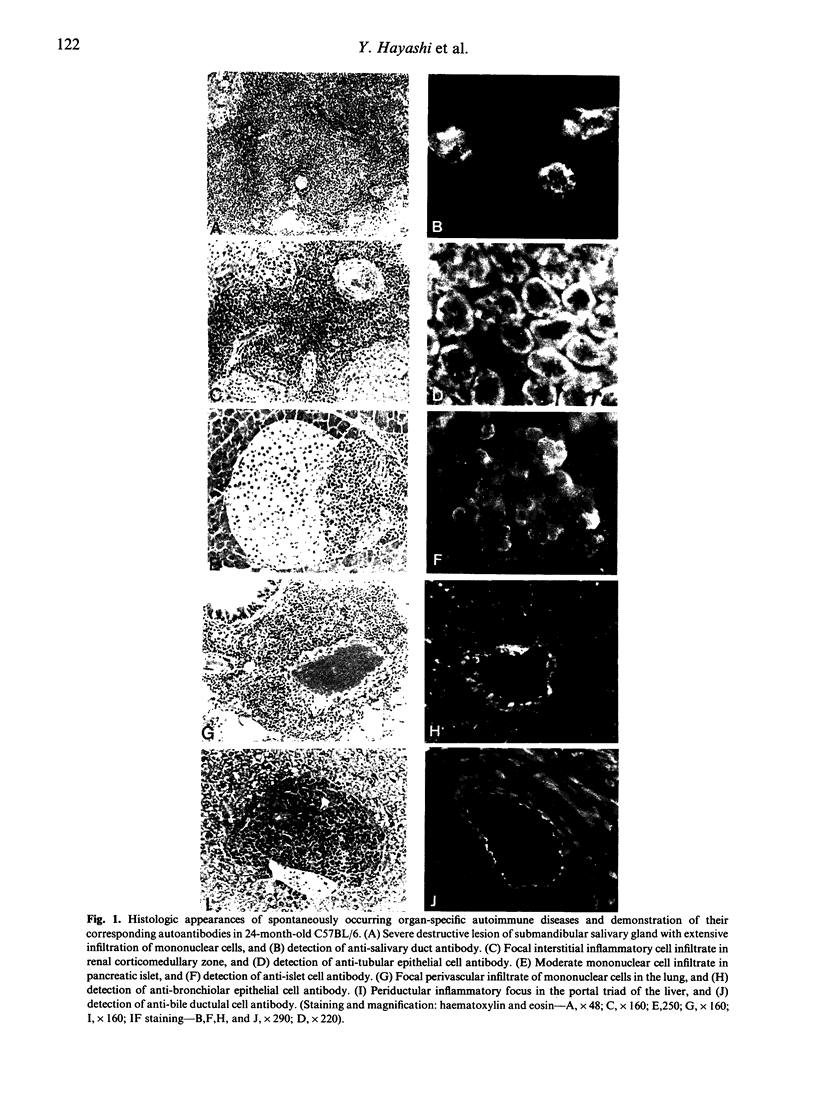
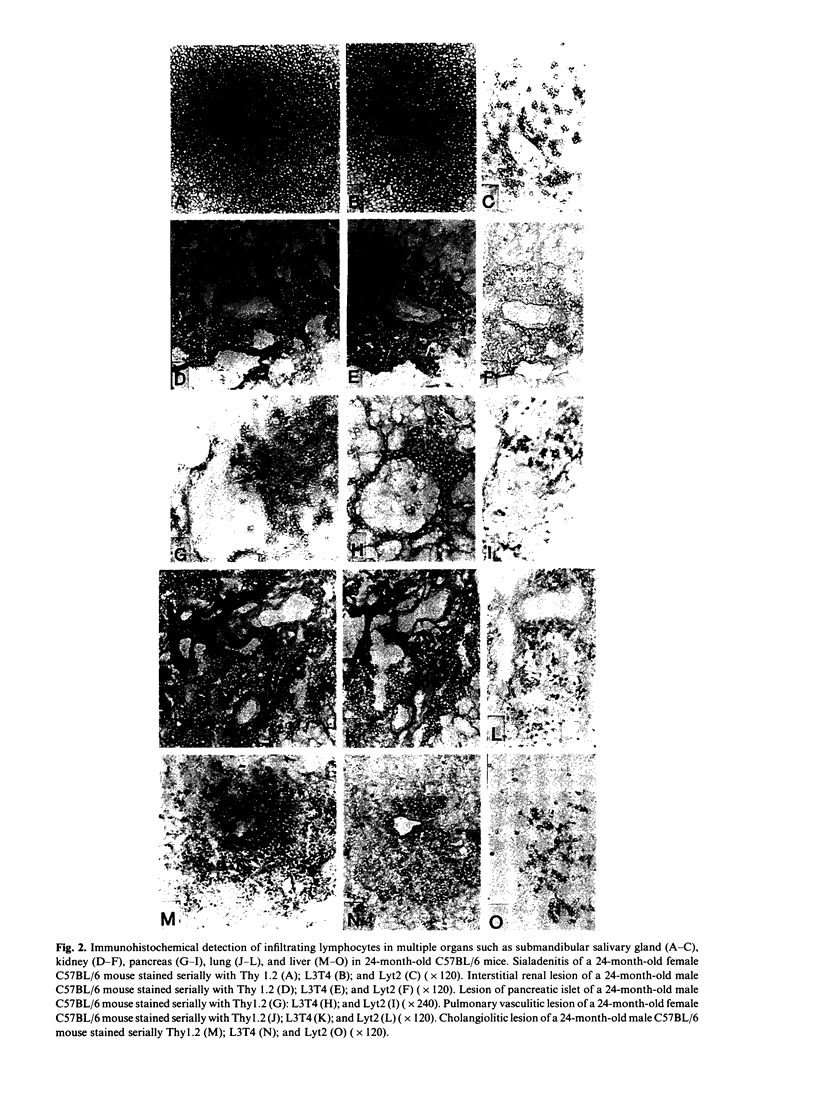
We have shown that spontaneously occurring, organ-specific autoimmune lesions develop in aged C57BL/6 mice of both sexes, especially in 24-month-old senescent mice. The inflammatory lesions were found in the multiple organs such as salivary gland, kidney, pancreas, lung, and liver, associated with ageing process. Organ-specific autoimmune lesions first appeared in 6-month-old C57BL/6 mice, and were aggravated with advancing age. In contrast, significant inflammatory changes did not develop in the thyroid, stomach, testis, ovary, and prostate in aged C57BL/6 mice. The incidence and severity of organ-specific autoimmune lesions in this strain of non-autoimmune mice increase with advance of age. The most severely affected lesion was sialadenitis developed in the submandibular salivary gland of aged mice, and a significant difference between male and female mice was noted only in the salivary gland. The infiltrating cells within the lesions of multiple organs consisted mainly of Thy 1.2+ and L3T4+ cells. Autoantibodies were detected in the sera of the mice with each corresponding organ-specific autoimmune lesions.
Full text
PDF

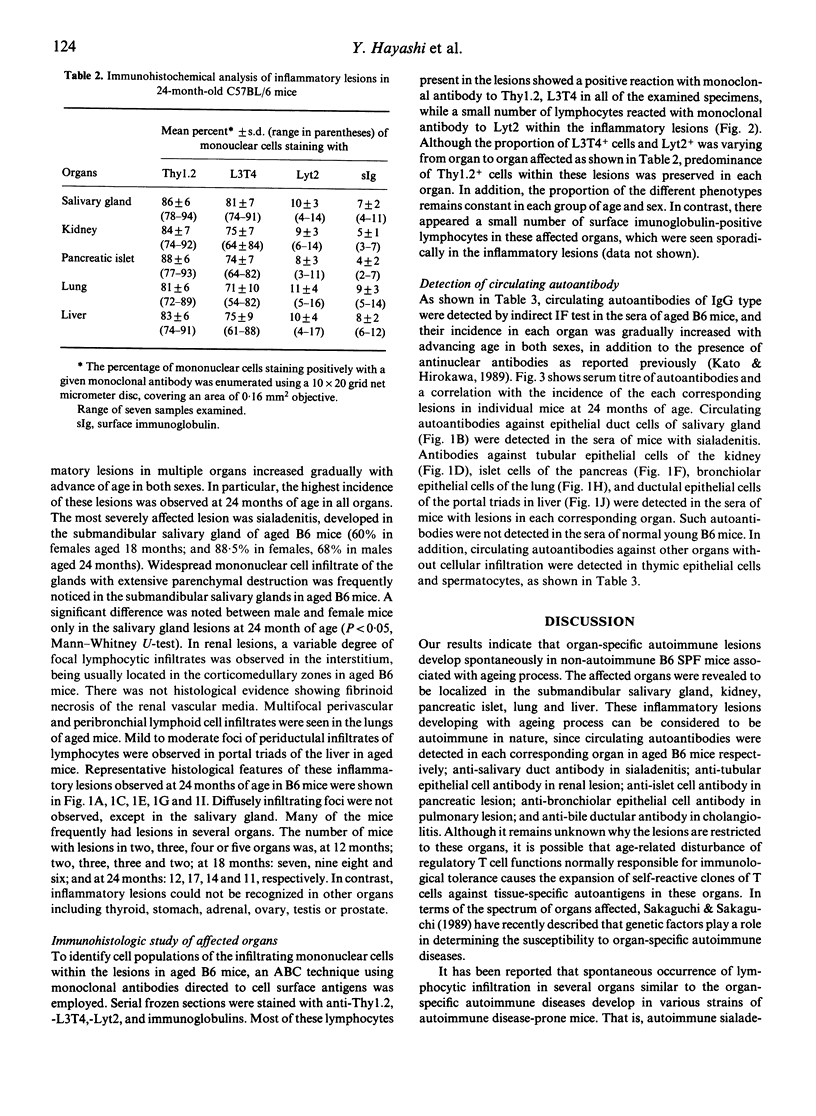
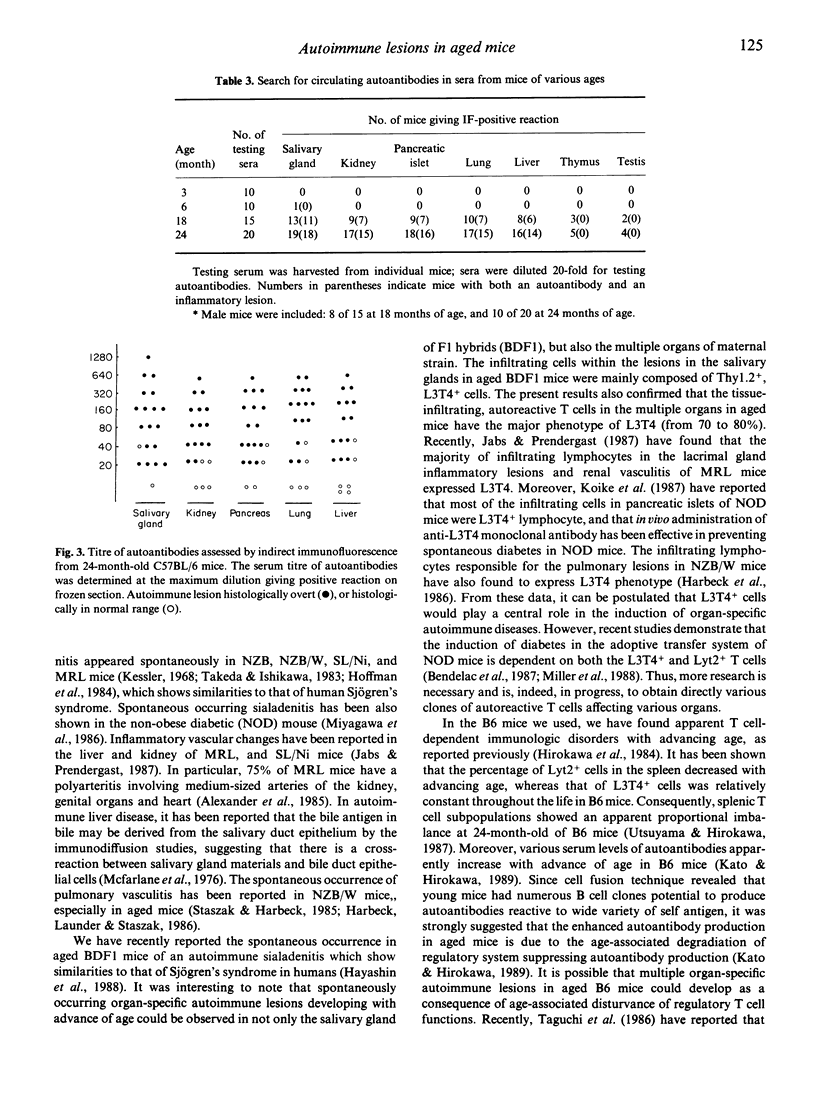

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Moyer C., Travlos G. S., Roths J. B., Murphy E. D. Two histopathologic types of inflammatory vascular disease in MRL/Mp autoimmune mice. Model for human vasculitis in connective tissue disease. Arthritis Rheum. 1985 Oct;28(10):1146–1155. doi: 10.1002/art.1780281011. [DOI] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan J. S., Gutman G. A., Weissman I. L., Talal N. Thymus-antigen- and immunoglobulin-positive lymphocytes in tissue infiltrates of NZB/NZW mice. Clin Immunol Immunopathol. 1974 Sep;3(1):16–31. doi: 10.1016/0090-1229(74)90020-8. [DOI] [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Dixon F. J. A spontaneous rheumatoid arthritis-like disease in MRL/l mice. J Exp Med. 1982 Jun 1;155(6):1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck R. J., Launder T., Staszak C. Mononuclear cell pulmonary vasculitis in NZB/W mice. II. Immunohistochemical characterization of the infiltrating cells. Am J Pathol. 1986 May;123(2):204–211. [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kurashima C., Utsuyama M., Hirokawa K. Spontaneous development of autoimmune sialadenitis in aging BDF1 mice. Am J Pathol. 1988 Jul;132(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Sato M., Hirokawa K. Induction of experimental allergic sialadenitis in mice. Am J Pathol. 1985 Mar;118(3):476–483. [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Yura Y., Yoshida H., Yanagawa T., Sato M. Development of allergic sialadenitis in mice immunized with mumps virus-infected submandibular salivary gland. Am J Pathol. 1986 May;123(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Hirokawa K., Utsuyama M., Goto H., Kuramoto K. Differential rate of age-related decline in immune functions in genetically defined mice with different tumor incidence and life span. Gerontology. 1984;30(4):223–233. doi: 10.1159/000212636. [DOI] [PubMed] [Google Scholar]
- Hoffman R. W., Alspaugh M. A., Waggie K. S., Durham J. B., Walker S. E. Sjögren's syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984 Feb;27(2):157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- Jabs D. A., Prendergast R. A. Reactive lymphocytes in lacrimal gland and vasculitic renal lesions of autoimmune MRL/lpr mice express L3T4. J Exp Med. 1987 Oct 1;166(4):1198–1203. doi: 10.1084/jem.166.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler H. S. A laboratory model for Sjögren's syndrome. Am J Pathol. 1968 Mar;52(3):671–685. [PMC free article] [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Kay M. M. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- McFarlane I. G., Wojcicka B. M., Tsantoulas D. C., Funk C., Portmann B., Eddleston A. L., Williams R. Cellular immune responses to salivary antigens in autoimmune liver disease with sicca syndrome. Clin Exp Immunol. 1976 Sep;25(3):389–395. [PMC free article] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Miyagawa J., Hanafusa T., Miyazaki A., Yamada K., Fujino-Kurihara H., Nakajima H., Kono N., Nonaka K., Tochino Y., Tarui S. Ultrastructural and immunocytochemical aspects of lymphocytic submandibulitis in the non-obese diabetic (NOD) mouse. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(3):215–225. doi: 10.1007/BF02899031. [DOI] [PubMed] [Google Scholar]
- Miyazawa M., Nose M., Kawashima M., Kyogoku M. Pathogenesis of arteritis of SL/Ni mice. Possible lytic effect of anti-gp70 antibodies on vascular smooth muscle cells. J Exp Med. 1987 Oct 1;166(4):890–908. doi: 10.1084/jem.166.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J Immunol. 1989 Jan 15;142(2):471–480. [PubMed] [Google Scholar]
- Staszak C., Harbeck R. J. Mononuclear-cell pulmonary vasculitis in NZB/W mice. I. Histopathologic evaluation of spontaneously occurring pulmonary infiltrates. Am J Pathol. 1985 Jul;120(1):99–105. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Takahashi T., Seto M., Namikawa R., Matsuyama M., Nishizuka Y. Development of multiple organ-localized autoimmune diseases in nude mice after reconstitution of T cell function by rat fetal thymus graft. J Exp Med. 1986 Jul 1;164(1):60–71. doi: 10.1084/jem.164.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ishikawa G. Experimental autoallergic sialadenitis in mice. Histopathological and ultrastructural studies. Virchows Arch A Pathol Anat Histopathol. 1983;400(2):143–154. doi: 10.1007/BF00585496. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Utsuyama M., Hirokawa K. Age-related changes of splenic T cells in mice--a flow cytometric analysis. Mech Ageing Dev. 1987 Sep 14;40(1):89–102. doi: 10.1016/0047-6374(87)90037-6. [DOI] [PubMed] [Google Scholar]