Abstract
Dendritic cells (DCs) are unique in their ability to stimulate T cells and initiate adaptive immunity. Injection of mice with the cytokine Flt3-ligand (FL) dramatically expands mature lymphoid and myeloid-related DC subsets. In contrast, injection of a polyethylene glycol-modified form of granulocyte/macrophage colony-stimulating factor (GM-CSF) into mice only expands the myeloid-related DC subset. These DC subsets differ in the cytokine profiles they induce in T cells in vivo. The lymphoid-related subset induces high levels of the Th1 cytokines interferon γ and interleukin (IL)-2 but little or no Th2 cytokines. In contrast, the myeloid-related subset induces large amounts of the Th2 cytokines IL-4 and IL-10, in addition to interferon γ and IL-2. FL- or GM-CSF-treated mice injected with soluble ovalbumin display dramatic increases in antigen-specific antibody titers, but the isotype profiles seem critically dependent on the cytokine used. Although FL treatment induces up to a 10,000-fold increase in ovalbumin-specific IgG2a and a more modest increase in IgG1 titers, GM-CSF treatment favors a predominantly IgG1 response with little increase in IgG2a levels. These data suggest that distinct DC subsets have strikingly different influences on the type of immune response generated in vivo and may thus be targets for pharmacological intervention.
Immune responses against T-cell-dependent antigens are heterogeneous with respect to the cytokines made by T-helper cells and the class of antibody secreted by B cells (1–5). Immune responses dominated by CD4+ T cells producing interferon (IFN) γ and B cells secreting IgG2a antibody are termed “Th1” responses; those dominated by CD4+ T cells producing interleukin (IL)-4 and IL-10 and B cells secreting IgG1 are termed “Th2” responses. At the individual T-cell level, considerable heterogeneity of cytokine profiles can be seen with T-cell clones, raising the possibility that the Th1 and Th2 global phenotypes may only represent two polar extremes of all possible single cell phenotypes (4). The type of cytokine produced early in an immune response appears to be key in determining whether a Th1-like or Th2-like immune response is generated. Thus, early production of IL-4 induces further IL-4 production in T cells whereas IL-12 induces IFNγ in T cells (1–5). However, the question of which cell types initiate the polarization of the response is less clear. The antigen-presenting cell, of which dendritic cells (DCs) are prime examples (6, 7), is thought to play a key role in determining the type of immune response (1–5). DCs, through production of IL-12 (8–10) may preferentially direct the development of Th1 cells in vitro (8) and in vivo (11) whereas other cells, such as B cells (12, 13) and NK T cells (14), may influence the development of Th2 responses.
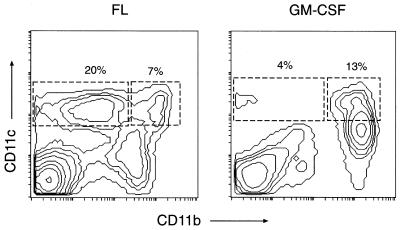
Attempts to study the regulation of immune responses by DCs have been impeded by their rarity in tissues. A recent solution to this problem has been the identification of DC growth factors such as Flt3-ligand (FL), which induce a profound expansion of mature DC subsets in mice (15, 16). The mature DC subsets generated in FL-treated mice all express high levels of CD11c and major histocompatibility complex class II, as well as CD86 and CD40, but differ in their levels of CD11b expression (15, 16) (Fig. 1). The majority of cells within the CD11c+ CD11bdull/− subset express high levels of CD8α and DEC-205, markers that are expressed on lymphoid-related DC subset in mice (17–21). Lymphoid-related DC have been shown to down-regulate the activation of T cells in vitro (20, 21) through a Fas-dependent mechanism (20). Cells within the CD11c+ CD11bbright subset do not express CD8α or DEC-205 but do express F4–80, 33D-1, and other myeloid-related markers, suggesting that they might be of myeloid origin (15, 16). These subsets are localized in distinct microenvironments, with the lymphoid-related subset residing in the T-cell zones and the myeloid-related subset residing in the marginal zones of the spleen (16, 21). Both subsets appear equally competent at stimulating antigen-specific T cell proliferation in vitro (E.M., B.P., C.M., K.B., E. Daro, M. Teepe, and H. McKenna, unpublished work). However, the lymphoid-related population can be induced to secrete much higher levels of biologically active IL-12 than the myeloid-related population (16), consistent with recent observations in normal mice (22). In this study, we investigate the ability of these DC subsets to prime antigen-specific T cells in vivo and examine the cytokine profiles that they induce in T cells.
Figure 1.
Relative expansion of DC subsets from FL- and GM-CSF-treated mice. Shown is the distribution of CD11c and CD11b on spleen cells from cytokine treated mice. CD11c+ CD11bbright (myeloid-related) and CD11c+ CD11b dull/− (lymphoid-related) subsets of DCs are defined as shown. Note that, in FL-treated mice, the lymphoid-related DCs are more prevalent than the myeloid-related DCs. Similar profiles are obtained with both C57BL/6 and BALB/c strains. Data are representative of a large number of experiments.
MATERIALS AND METHODS
Mice.
All mice were housed in a specific-pathogen-free facility. BALB/c and C57BL/6 mice were purchased from The Jackson Laboratory; a male DO11.10/SCID mouse was received from the laboratory of Marc Jenkins (University of Minnesota) and was bred to BALB/c/SCID mice in house. For adoptive transfers, age- and sex-matched recipients were injected i.v. with 2 × 106 T cells as described (23).
Injections.
Mice were injected s.c. with 10 μg of recombinant human FL (human Chinese hamster ovary cell-derived produced at Immunex) for 9 consecutive days. Recombinant granulocyte/macrophage colony-stimulating factor (GM-CSF) was modified with polyethylene glycol at Immunex and was injected s.c. (5 μg per day) for 5 consecutive days. In adjuvant studies, chicken ovalbumin (Sigma) was freshly prepared in PBS, was filtered, and was injected s.c.
DCs.
DC subsets from FL- and GM-CSF-treated mice were sorted by flow cytometry as described (15, 16). DCs were incubated with ovalbumin 323–339 (OVA[323–339]) peptide at 4°C for 2 hours, were washed, and were injected into the footpads of reconstituted mice (3 × 105 DCs per footpad).
In Vitro Cultures.
Popliteal lymph node cells (5 × 105 cells) were plated in triplicate in 200 μl of DMEM complete supplemented with 5% fetal bovine serum, together with different concentrations of OVA[323–339]. Proliferative responses were assessed after 72 hours of culture in a humidified atmosphere of 5% CO2 in air. Cultures were pulsed with 0.5 μCi tritiated thymidine [3H] for 5 hours, and the cells were harvested onto glass fiber sheets for counting on a gas-phase β counter. For cytokine assays, aliquots of culture supernatants were removed after 72 hours and were assayed for the presence of IFNγ, IL-2, IL-4, and IL-10 by ELISAs adapted from PharMingen protocols.
Measurement of Ovalbumin-Specific Serum Titers.
Ninety-six-well ELISA plates (Maxisorp, Nunc) were coated overnight with 1 μg/well ovalbumin in PBS at 4°C, were blocked with PBS/5% fetal bovine serum, and washed with PBS/0.1% Tween-20. Serum samples were diluted in PBS/5% fetal bovine serum (starting at 1/100), and threefold dilutions were made. Plates were incubated for 2 hours at room temperature, were washed, and were incubated with alkaline phosphatase-conjugated anti-IgG1 (1/2,000; PharMingen), anti-IgG2a, anti-IgG2b, or anti-IgM (1/1,000; PharMingen) detecting antibodies for an additional 2 hours at room temperature. Plates were washed again, and enzyme activity was detected with p-nitrophenyl phosphate disodium (Sigma). The amount of reaction product was assessed on an ELISA plate reader at an OD of 405 nm by using the deltasoft program (DeltaPoint, Monterey, CA). Multiple-point analysis was performed on each set of isotype titrations by using the bioassay program (Immunex), selecting a maximum value for each isotype and determining for each sample the dilution giving half-maximal OD value, thus generating arbitrary unit-per-milliliter values as described (24).
RESULTS
Lymphoid and Myeloid-Related DCs from FL-Treated Mice Prime T Cells in Vivo Equally Efficiently.
To investigate the ability of these DC subsets to prime antigen-specific T cells in vivo, we used T-cell receptor (TCR) transgenic mice that contained rearranged TCRα and TCRβ genes in their germline DNA encoding a TCR specific for the peptide fragment OVA [323–339] bound to I-Ad class II major histocompatibility complex molecules (DO11.10 mice) (25). The transgenic TCR could be detected with the KJ126 mAb that binds only to this particular TCR heterodimer (26). Because DO11.10 T cells contain a small subpopulation of cells with a memory phenotype, presumably because of activation through a second endogenously rearranged TCR (27), all of our experiments were performed with DO11.10/SCID mice, which do not contain cells with this memory phenotype (26). TCR transgenic T cells from DO11.10/SCID mice were adoptively transferred into syngeneic BALB/c recipients, such that they constituted a small (<0.3%) but detectable proportion of all T cells as described (23, 24, 28).
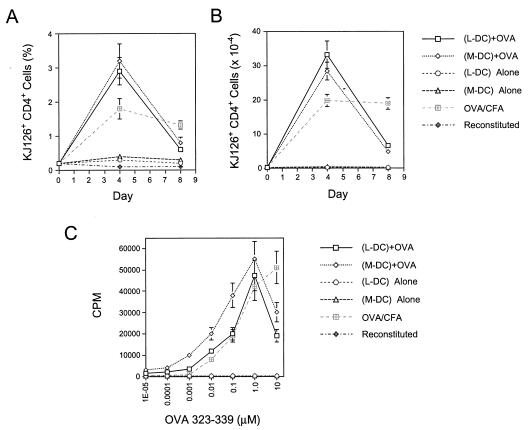
Lymphoid-related DC and myeloid-related DC subsets from spleens of FL-treated mice were isolated by flow cytometry, were loaded with OVA[323–339] in vitro, and were injected into the footpads of T-cell-reconstituted mice. The ensuing CD4+ OVA-specific T-cell response was monitored by flow cytometry. Both DC populations could prime T cells in an antigen-specific manner (Fig. 2 A and B), with similar kinetics and magnitude. Thus, 4 days after priming, at the height of the immune response, the percentage of KJ126+ CD4+ T cells was ≈3% in the draining lymph nodes of mice primed with either the lymphoid or myeloid DC subset; by day 8, the percentage in both groups of mice had almost returned to baseline levels. The absolute numbers of KJ126+ CD4+ T cells in the draining lymph nodes reflect this kinetics.
Figure 2.
(A and B) Kinetics of expansion of antigen-specific (KJ126+ CD4+), transgenic T cells in mice primed with lymphoid-related (L-DC; CD11c+ CD11bdull/−) and myeloid-related (M-DC; CD11c+ CD11bbright) DC subsets from FL-treated mice. Sorted DCs were loaded with OVA[323–339] in vitro and were injected into the footpads of mice reconstituted with transgenic T cells. T-cell expansion was measured by flow cytometric analysis of lymph node cells at various time points. Data were pooled from six independent experiments with a total of 18–21 lymph nodes per time point. (C) In vitro restimulation of antigen-specific T cells expanded in vivo, with varying concentrations of OVA[323–339] peptide. In vitro lymph node cultures were set up 4 days after in vivo priming, were pulsed with [H]3 72 hrs later, and were mashed after 5 hrs.
At the height of the response, 4 days after priming, the popliteal lymph nodes were harvested, and single-cell suspensions were prepared and cultured in vitro with various concentrations of OVA[323–339] to assess the in vitro proliferative capacity of antigen-specific T cells. T cells that had been primed in vivo by either the lymphoid- or myeloid-related DCs could proliferate efficiently in vitro on restimulation with antigen; however, there was a 2- to 5-fold increase in the sensitivity of antigen-specific T-cell proliferation in T cells that had been primed in vivo with the myeloid DC, compared with those primed with the lymphoid-related DC subset (Fig. 2C).
Lymphoid- and Myeloid-Related DCs from FL-Treated Mice Exert Differential Effects on Cytokine Production in Antigen-Specific T Cells in Vivo.
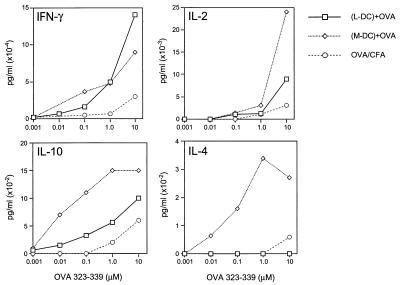
Cytokine production by antigen-specific T cells primed by the lymphoid or myeloid-related DC subsets was measured by assaying the culture supernatants from the cultures described above for IFNγ, IL-2, IL-10, and IL-4. Assessment of cytokine production in these cultures revealed that there were no significant differences in IFNγ and IL-2 production in T cells primed by either DC subset; however, the myeloid-related DC subset always induced much greater levels of IL-10 and IL-4 production (Fig. 3). Thus, the lymphoid-related subset induced no detectable IL-4 whereas the myeloid-related subset induced high levels of IL-4. Furthermore, the T cells primed by the myeloid-related DC subset exhibited a >100-fold increase in the antigen dose-dependent sensitivity of IL-10 production, compared with T cells primed by the lymphoid-related DC subset.
Figure 3.
Cytokine production by antigen-specific T cells restimulated in vitro with varying concentrations of OVA[323–339]. In vitro lymph node cultures were set up 4 days after priming, and supernatants were assayed 72 hours later by cytokine ELISA. Data are representative of seven independent experiments.
GM-CSF Treatment Preferentially Expands a Myeloid-Related DC Subset, Which Resembles the Myeloid DC in FL-Treated Mice.
Administration (5 days) of a chemically modified (pegylated) form of GM-CSF (which exhibits an extended biological half-life, compared with underivatized GM-CSF) into mice preferentially expands the myeloid-related DC (CD11c+ CD11bdull/−) subset (Fig. 1). This myeloid-related DC subset appears to be similar to that generated by FL-treatment, in so far as it failed to express detectable CD8α or DEC-205 (E.M., B.P., C.M., K.B., E. Daro, M. Teepe, and H. McKenna, unpublished work), which have been shown to be expressed selectively on lymphoid-related DC (15, 16, 19). They are also able to prime naive, antigen-specific T cells in vitro as efficiently as the myeloid or lymphoid-related DC subsets from FL-treated mice (E.M., B.P., C.M., K.B., E. Daro, M. Teepe, and H. McKenna, unpublished work).
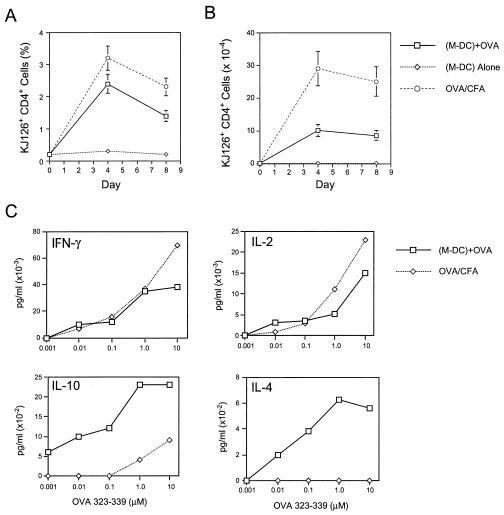
Similiar experiments with the sorted DC subset from GM-CSF-treated mice revealed that this putative myeloid-related population also could prime OVA-specific T cell expansion as efficiently as the lymphoid and myeloid DC populations from FL-treated mice (Fig. 4 A and B). As observed previously with the myeloid-related population from FL treated mice, there was significant production of IL-4 and IL-10 by the OVA-specific T cells after restimulation with OVA[323–339] in vitro (Fig. 4C). These data support the notion the distinct DC subsets prime T cells equally efficiently but elicit different cytokine profiles in antigen-specific T cells in vivo.
Figure 4.
(A and B) Kinetics of expansion of antigen-specific (KJ126+ CD4+), transgenic T cells in mice primed with the myeloid DC (M-DC) subset in GM-CSF-treated mice. (C) Cytokine production by antigen-specific T cells restimulated in vitro with varying concentrations of OVA[323–339]. In vitro cultures were set up 4 days after priming, and supernatants were assayed 72 hours later. Data are representative of two independent experiments.
FL and GM-CSF Dramatically Enhance Antibody Production to Soluble Antigens but Differ in the Class of Antibodies They Induce.
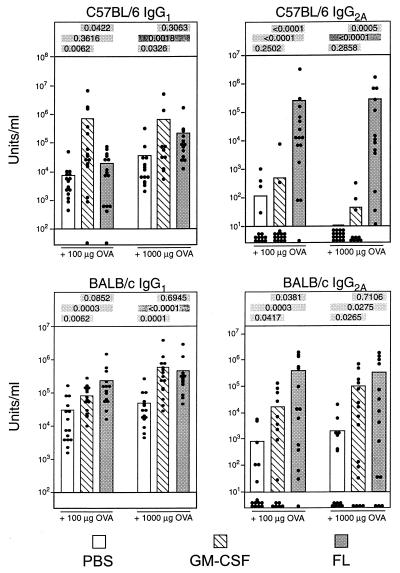
To determine whether these differences in T-cell cytokine profiles could influence the class of antibody produced in vivo, we performed the following experiment. Cohorts of BALB/c or C57BL/6 mice were treated with either FL or GM-CSF for 9 and 5 days, respectively, to induce maximal expansion of DCs. The effects of FL and GM-CSF on expanding DC subsets in vivo does not appear to depend on the strain of mice used (data not shown). On the final day of cytokine treatment, mice were injected s.c. with various doses of ovalbumin in PBS. Twenty-one days later, identical immunizations were performed with no cytokine treatment. One week later (day 28), mice were bled, and the ovalbumin-specific antibody titers were determined by ELISA. Both FL and GM-CSF significantly enhanced the ovalbumin-specific antibody titers of all isotypes analyzed (Fig. 5) compared with the ovalbumin-immunized, PBS-treated control mice. Consistent with our recent observations (24), FL treatment resulted in a >10,000-fold increase in IgG2a titers but a much more modest increase in IgG1 titers over PBS-treated controls. However, GM-CSF treatment resulted in a much weaker IgG2a response than FL-treatment, and this was more apparent in the C57BL/6 strain, in which GM-CSF failed to stimulate IgG2a (Fig. 5). In contrast, GM-CSF stimulated significant levels of IgG1, and, in some cases, the levels of IgG1 stimulated were significantly higher than those in FL-treated mice.
Figure 5.
Adjuvant effects of FL and GM-CSF in ovalbumin-immunized mice. Shown are antigen-specific, secondary serum antibody titers in FL- and GM-CSF-treated mice after challenge with ovalbumin. PBS- or cytokine-treated mice were primed and boosted with ovalbumin, and secondary anti-ovalbumin antibody responses were measured by ELISA. Each dot represents a single mouse. Data are pooled from three independent experiments, each of which had five mice per group. Because the data are not normally distributed, the Wilcoxon rank sum 2-sided test has been used to analyze the P values. P values comparing the differences between the PBS, GM-CSF, and FL groups are shown above each graph.
The data in Fig. 5 are pooled from three independent experiments, each of which had five mice per group. The variability in the IgG2a OVA-specific antibody titers in mice treated with FL or GM-CSF is caused largely by variability between individual experiments. The source of this experiment to experiment variation is not known. Nevertheless, each experiment showed exactly the same trends between groups; compared with GM-CSF- and PBS-treated mice, FL-treated mice not only displayed much larger titers of IgG2a OVA-specific antibody but also had much higher frequency of responders. This is evident from the means of the graphs as well as with differences in the numbers of nonresponders within each group. The nonresponders are stacked at the bottom of the graph for each group. Thus, although both FL and GM-CSF acted as adjuvants, there were class-specific differences in the antibody profiles generated by each cytokine, with FL giving a predominantly IgG2a response and GM-CSF favoring an IgG1 response. These results reflect the differences in cytokine profiles observed in T cells stimulated by the distinct DC subsets.
DISCUSSION
The results presented in this paper suggest that distinct DC subsets may differentially regulate the Th1/Th2 balance of an immune response in vivo. Although both the lymphoid and myeloid-related DC subsets can prime antigen-specific T cells equally efficiently, they induce distinct cytokine profiles in T cells. The lymphoid-related DCs induce the Th1 cytokines IFNγ and IL-2 whereas the myeloid-related DC subset induces high levels of the Th2 cytokines IL-4 and IL-10, in addition to the Th1 cytokines.
Mechanism of T-Cell Cytokine Skewing by Distinct DC Subsets.
The precise mechanism by which these DC subsets mediate their effects is, at present, unclear but may well relate to potential differences in the repertoire of costimulatory molecules expressed by the subsets. The difference is unlikely to be accounted for by the B7 molecules, by CD40, or by the levels of class II major histocompatibility complex–peptide complexes on the surfaces because the myeloid- and lymphoid-related DC subsets in FL-treated mice express similar levels of all of these molecules (4). However, potential differences in the expression of costimulatory molecules such as OX-40 ligand [which may stimulate IL-4 production in T cells (29)] may account for the functional dichotomy of the two subsets. Another factor that could contribute to this dichotomy is the cytokines made by the DCs themselves. Lymphoid-related DCs can be induced to secrete higher levels of biologically active IL-12 than myeloid DCs (16, 22). Therefore, the former may have induced greater IFNγ production in T cells than the latter. However, our experiments suggest that even the relatively low levels of IL-12 produced by the myeloid-related DC subset is sufficient to induce abundant IFNγ production in T cells. Clearly the expression of Th2-inducing cytokines such as IL-4 (1) and IL-6 (30) by the DC subsets warrants further investigation.
The question of whether the DC subsets interact directly with the T cells in vivo or whether they transfer antigen to a third cell type is intriguing, particularly given the resurgent interest in the phenomenon of “cross-priming” (31, 32). Thus, it is possible that antigen is transferred from the exogenous DC subset to some endogenous APC population, which then may present it to T cells. If the lymphoid and myeloid DC subsets differed in their abilities to hand over antigen to other antigen-presenting cells, this potentially could lead to differences in T-cell priming or in the cytokines made by the T cells.
Adjuvant Effects of FL and GM-CSF.
Whatever the mechanism, the differential cytokine skewing in T cells by distinct DC subsets appears to result in strikingly different antibody profiles being elicited by FL and GM-CSF (Fig. 5). In C57BL/6 mice, GM-CSF (which preferentially expands the myeloid DC subset; Fig. 1) elicits significant increases in IgG1 titers but no IgG2a production (Fig. 5). In contrast, FL (which expands both the lymphoid and myeloid DC subsets, at a numeric ratio of ≈3:1; Fig. 1) elicits modest increases in IgG1 titers but dramatic increases in IgG2a titers (Fig. 5). These differences in antibody profiles are consistent with the cytokine profiles induced in T cells. Lymphoid-related DCs would prime T cells to become Th1 T cells, which enhance IgG2a secretion by B cells. Myeloid-related DCs would prime T cells to make abundant IL-4 and IL-10 (in addition to IFNγ and IL-2), and it is likely that the Th2 cytokines would mitigate the effects of IFNγ, thus favoring an IgG1 response. Similar though less striking trends are observed in BALB/c mice. This strain difference may be attributed to potential differences in production of IL-18 (33) or IL-1α by the DC subsets in the different strains. Both IL-18 and IL-1α can potentiate IL-12-induced Th1 development in BALB/c but not C57BL/6 mouse strains (34).
Perspectives.
These data are consistent with recent observations (35) that indicate that lymphoid and myeloid DCs in normal mice also can induce distinct cytokine profiles in T cells. Both studies suggest that the lymphoid and myeloid DCs prime T cells equally efficiently in vivo. We currently are investigating whether lymphoid-related DCs could tolerize CD4+ T cells in vivo via a Fas-dependent mechanism (20). One possibility is that CD4+ T cells may be tolerized in vivo by repeated stimulation with lymphoid- but not myeloid-related DC. Indeed, the 2- to 5-fold less efficient in vitro stimulation of T cells, which were primed in vivo by the lymphoid DCs (Fig. 2C), may represent a dampening of the T-cell response by activation-induced cell death. In this context, it is worth noting that activated Th1 cells, rather than Th2 cells, are susceptible to Fas-mediated apoptosis (36, 37). Therefore, the lymphoid and myeloid DC subsets initially may prime T cells equally efficiently, but, on repeated stimulation by the lymphoid DCs, the resulting Th1 T cells may be more susceptible to Fas-mediated apoptosis.
Finally, the functional differences of the DC subsets described here should be viewed in the context of their geographical separation in lymphoid microenvironments. In the spleen, the lymphoid and myeloid DC subsets are localized in the T-cell zones and the marginal zones, respectively (16). The possibility that these subsets may be exposed to different types of antigens because of their geographical isolation and differences in their cell-surface markers must now be investigated.
In summary, our data suggest that the class of immune response generated in vivo can be controlled differentially by distinct DC subsets. This may provide a unique opportunity to elicit optimally effective immunity in different clinical settings by using distinct DC subpopulations. Thus, in various autoimmune diseases or in Helicobacter pylori infections, the prospect of using myeloid-related DC or GM-CSF to vaccinate against pathogenic Th1 responses may be attractive. In contrast, lymphoid-related DCs that promote protective Th1 responses may hold great promise in tumor immunotherapy, antiviral immunity, and in allergy.
Acknowledgments
We thank Dr. Marc Jenkins for advice and DO11.10/SCID mice; Dr. Muriel Moser and Roberto Maldanado for sharing unpublished data; Dr. Nancy Nightlinger for synthesis of pegylated GM-CSF; Dr. Alan Alpert, Daniel Hirschstein, and Steve Braddy for help with flow cytometry; Anne Aumell for editorial assistance; Dr. Jacques Banchereau for advice and discussion; and Drs. Ken Shortman, Gus Nossal, Muriel Moser, Doug Williams, Hilary McKenna, and Laurent Galibert for critical comments on the manuscript.
ABBREVIATIONS
- IFN
interferon
- IL
interleukin
- DC
dendritic cell
- FL
Flt3-ligand
- TCR
T cell receptor
- GM-CSF
granulocyte/macrophage colony-stimulating factor
References
- 1.Seder R A, Paul W E. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 2.O’ Garra A. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann T R, Sad S. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 4.Kelso A. Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 5.Constant S L, Bottomly K. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 6.Steinman R M. Annu Rev Immunol. 1983;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman R M. Nature (London) 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C-Y, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O’Garra A. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 9.Koch F, Stanzl U, Jennewin P, Janke K, Heufler E, Kampgen E, Romani N, Schuler G. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sornasse T, Flamand V, De Becker G, Bazin H, Tielmans F, Thielemans K, Urbain J, Leo O, Moser M. J Exp Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macaulay A E, DeKruyff R H, Goodnow C C, Umetsu D T. J Immunol. 1997;158:4171–4179. [PubMed] [Google Scholar]
- 13.Stockinger B, Zal T, Zal A, Gray D. J Exp Med. 1996;183:891–899. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendelac A, Rivera M N, Park S-H, Roark J H. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 15.Maraskovsky E, Brasel K, Teepe M, Roux E R, Lyman S D, Shortman K D, McKenna H J. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulendran B, Lingappa J, Kennedy M K, Smith J, Teepe M, Rudensky A, Maliszewski C R, Maraskovsky E. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 17.Ardavin C, Wu L, Shortman K. Nature (London) 1993;362:761–764. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Li C L, Vremec D, Ardavin K, Winkel K, Suss G, Georgiou H, Maraskovsky E, Cook W, Shortman K. Eur J Immunol. 1995;25:418–429. doi: 10.1002/eji.1830250217. [DOI] [PubMed] [Google Scholar]
- 19.Vremec D, Shortman K. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 20.Suss G, Shortman K. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman R M. J Exp Med. 1997;186:665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis e Sousa C, Hieny S, Scharton-Kerston T, Jankovic D, Charest H, Germain R N, Sher A. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 24.Pulendran B, Smith J L, Jenkins M, Schoenborn M, Maraskovsky E, Maliszewski C. J Exp Med. 1998;188:2075–2082. doi: 10.1084/jem.188.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 26.Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee W T, Cole-Calkins J, Street N E. J Immunol. 1996;157:5300–5307. [PubMed] [Google Scholar]
- 28.Khoruts A, Mondino A, Pape K A, Reiner S L, Jenkins M K. J Exp Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn S, Toellner K-M, Raykundalia C, Goodall M, Lane P. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurts C, Heath W R, Carbone F, Allison J, Miller J F A P, Kosaka H. J Exp Med. 1996;182:885–889. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bevan M J. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamura H, Tsutsul H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Mukuda Y, Hattori K, et al. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 34.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, et al. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 35.Maldanado, R., DeSmedt, T., Michel, P., Godfroid, J., Pajak, B., Heirman, C., Thielman, K., Leo, O., Urbain, J. & Moser, M. (1999) J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 36.Ramsdell F, Seaman M S, Miller R E, Picha K S, Kennedy M K, Lynch D H. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Brunner T, Carter L, Dutton R W, Rogers P, Bradley L, Sato T, Reed J C, Green D, Swain S L. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]