Abstract
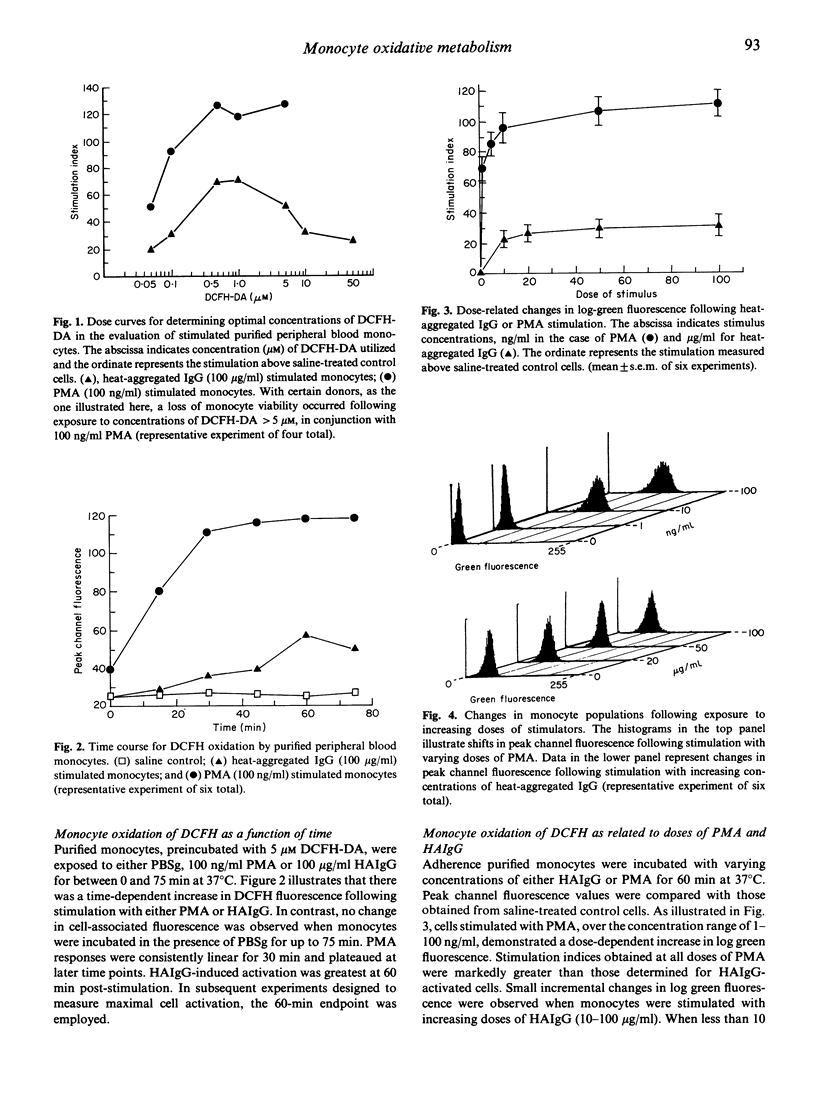
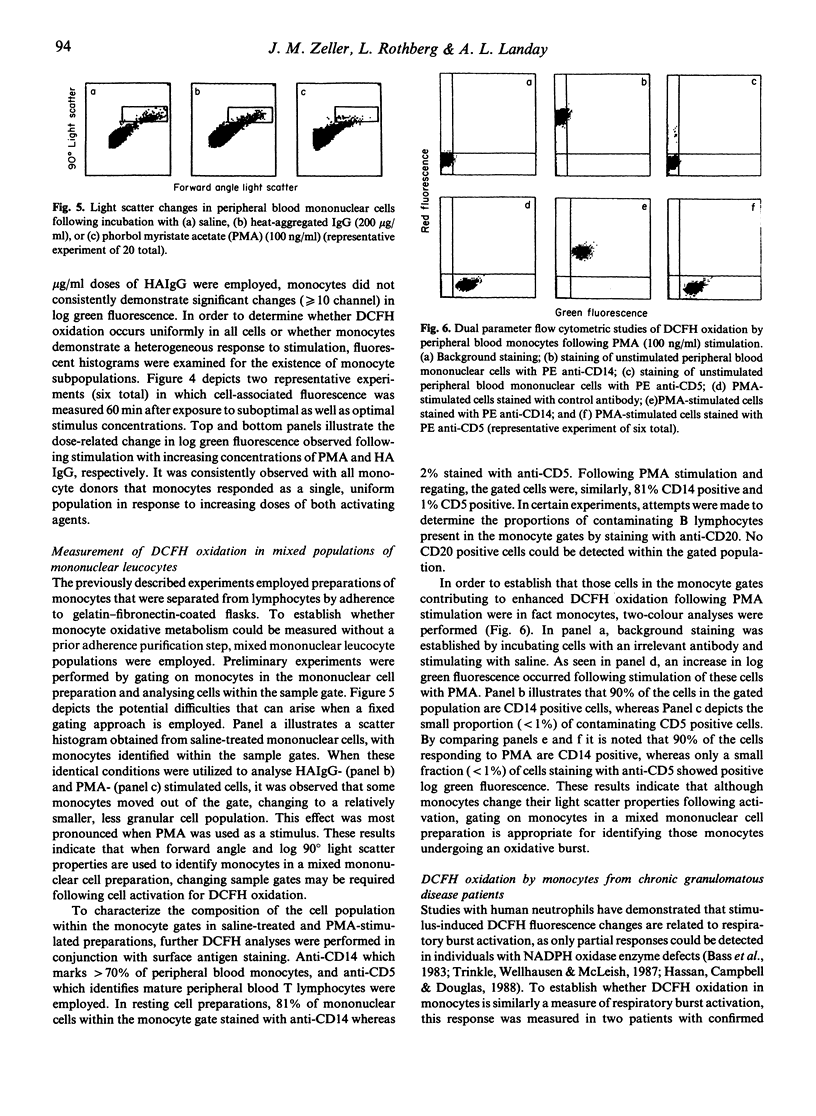
Assays routinely employed to evaluate human monocyte respiratory burst activation have been limited to measuring responses of bulk cell preparations. We demonstrate that individual monocyte responses can be easily assessed by using 2',5' dichlorofluorescin diacetate (DCFH-DA) and flow cytometry. Adherence purified monocytes were incubated with DCFH-DA, washed, and stimulated with either phorbol myristate acetate (PMA) or heat-aggregated IgG (HAIgG). Log green fluorescence signals were measured by using a flow cytometer equipped with a 5-W argon laser set at an excitation wavelength of 488 nm. Optimal conditions for stimulation included exposure to 5 microM concentrations of DCFH-DA for 15 min, followed by a 60-min incubation with either PMA or HAIgG. Dichlorofluorescin (DCFH) oxidation by monocytes increased in a graded fashion as a function of stimulus concentration. Monocytes responded as a uniform population in response to increasing doses of PMA and HAIgG. This oxidative response was also monitored in mixed populations of mononuclear leukocytes, with monocytes identified on the basis of light scatter properties and surface antigen staining with anti-CD14. More than 90% of cells demonstrating increases in log green fluorescence signals following activation were CD14 positive. Measurement of DCFH oxidation by monocytes is reflective of the capacity to undergo a respiratory burst response, in that monocytes obtained from patients with chronic granulomatous disease were only minimally reactive. This assay, representing a rapid means of assessing monocyte respiratory burst activation by single cell analysis, is suitable for use in both clinical and research settings.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986 Feb 1;136(3):860–866. [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Bender J. G., Van Epps D. E., Steinkamp J. A. Characterization of human mononuclear cells using reduced pyridine nucleotide fluorescence and flow cytometry. J Leukoc Biol. 1985 Nov;38(5):603–611. doi: 10.1002/jlb.38.5.603. [DOI] [PubMed] [Google Scholar]
- Braun D. P., Kessler H., Falk L., Paul D., Harris J. E., Blaauw B., Landay A. Monocyte functional studies in asymptomatic, human immunodeficiency disease virus (HIV)-infected individuals. J Clin Immunol. 1988 Nov;8(6):486–494. doi: 10.1007/BF00916955. [DOI] [PubMed] [Google Scholar]
- De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985 Apr 22;78(2):323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Campbell D. E., Douglas S. D. Phorbol myristate acetate induced oxidation of 2',7'-dichlorofluorescin by neutrophils from patients with chronic granulomatous disease. J Leukoc Biol. 1988 Apr;43(4):317–322. doi: 10.1002/jlb.43.4.317. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Rajkovic I. A., Williams R. Rapid microassays of phagocytosis, bacterial killing, superoxide and hydrogen peroxide production by human neutrophils in vitro. J Immunol Methods. 1985 Apr 8;78(1):35–47. doi: 10.1016/0022-1759(85)90327-8. [DOI] [PubMed] [Google Scholar]
- Robinson J. P., Bruner L. H., Bassoe C. F., Hudson J. L., Ward P. A., Phan S. H. Measurement of intracellular fluorescence of human monocytes relative to oxidative metabolism. J Leukoc Biol. 1988 Apr;43(4):304–310. doi: 10.1002/jlb.43.4.304. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Disorders of phagocyte function. Annu Rev Immunol. 1987;5:127–150. doi: 10.1146/annurev.iy.05.040187.001015. [DOI] [PubMed] [Google Scholar]
- Ruch W., Cooper P. H., Baggiolini M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983 Oct 28;63(3):347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- Shiotsuki K., Ohta M., Hiyoshi Y., Honda J., Hirata T., Yasaka T., Yokoyama M. M. Functional heterogeneity of human monocytes--with a special reference to flow cytometric assays. J Clin Lab Immunol. 1987 Sep;24(1):45–50. [PubMed] [Google Scholar]
- Zeller J. M., Landay A. L., Lint T. F., Gewurz H. Enhancement of human peripheral blood monocyte respiratory burst activity by aggregated C-reactive protein. J Leukoc Biol. 1986 Dec;40(6):769–783. doi: 10.1002/jlb.40.6.769. [DOI] [PubMed] [Google Scholar]


