Abstract
Previous in vitro studies suggest that transcription of the unrearranged immunoglobulin switch region and its 5' flanking region precedes isotype switching. These transcripts, which are devoid of a variable region, contain unique exons and are called germ-line (GL) mRNA. A crucial point in this regard is whether such transcripts could be detected in vivo, and if their expression correlates with immunoglobulin class switching in health and disease. To understand the in vivo role of this transcriptional activity we have adapted the reverse transcription-polymerase chain reaction (PCR) to analyse the GL transcripts from unstimulated peripheral blood mononuclear cells (PBMC) in healthy individuals and in different immunological diseases. Furthermore, mononuclear cells from different human organs were also analysed. We report here that GL (I alpha, I gamma and I epsilon used to designate the GL mRNA for IgA, IgG and IgE, respectively) mRNA are expressed differentially during ontogeny of B cells. Unexpectedly, no difference of I alpha mRNA expression between the PBMC and the secondary lymphoid organs was detected. Rather, the levels of GL transcripts were correlated to the number of sIgM+ cells. GL mRNA of all three isotypes could be detected in PBMC from healthy donors, whereas there was a decrease of specific GL transcript synthesis in individuals with immunoglobulin deficiency. Furthermore, during the in vivo immune response in a parasitic infection, we could demonstrate an induction of GL I epsilon mRNA during in vivo immune response. Concomitantly, there was also increased synthesis of productive epsilon transcripts. These findings implicate a potential role of GL transcription during in vivo immunoglobulin class switching.
Full text
PDF
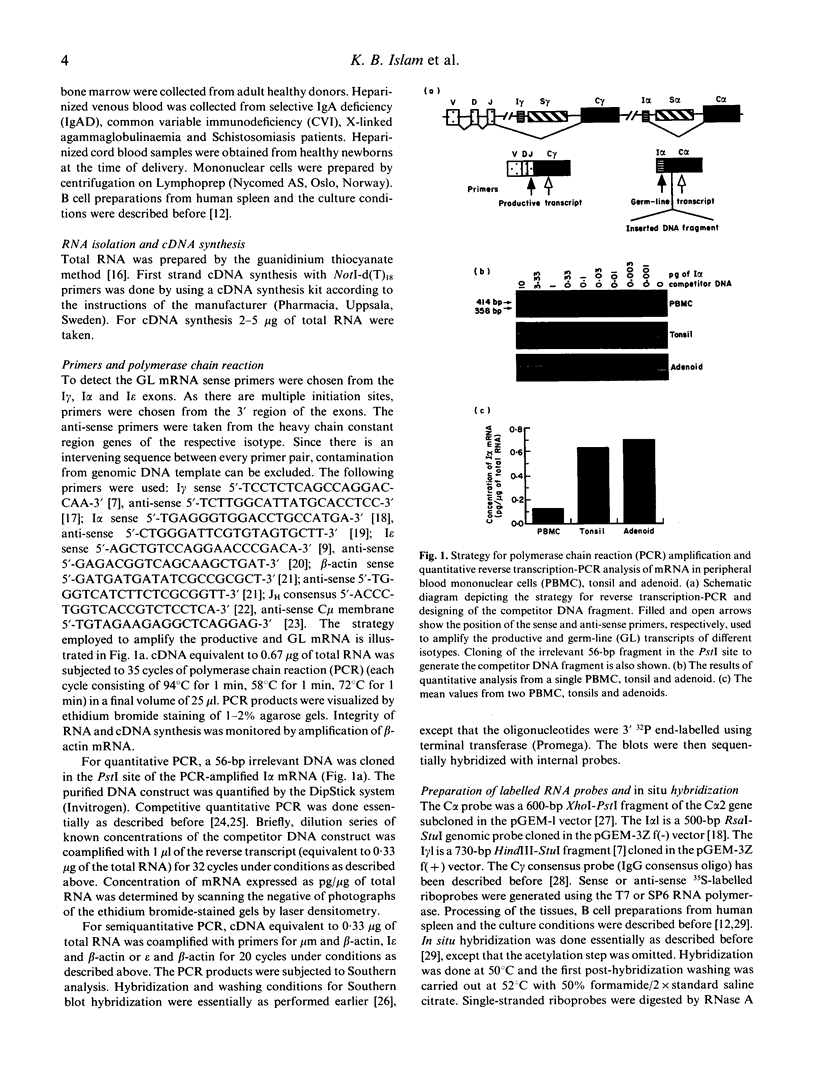

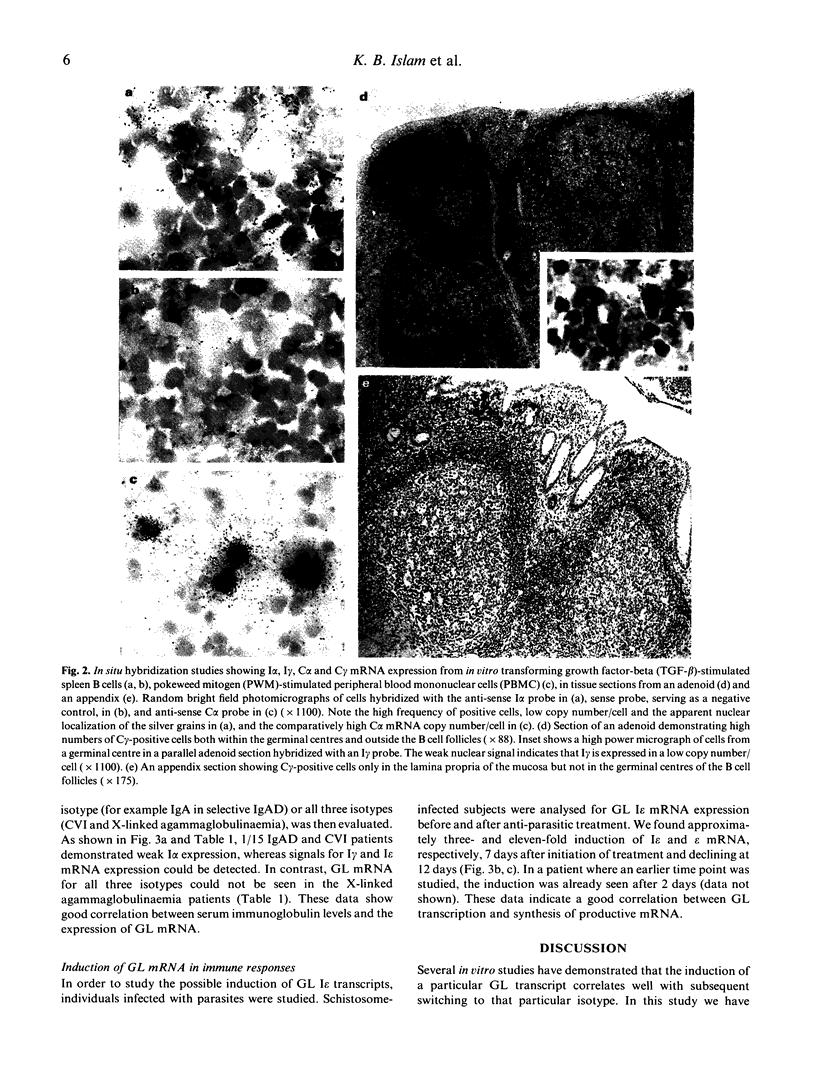
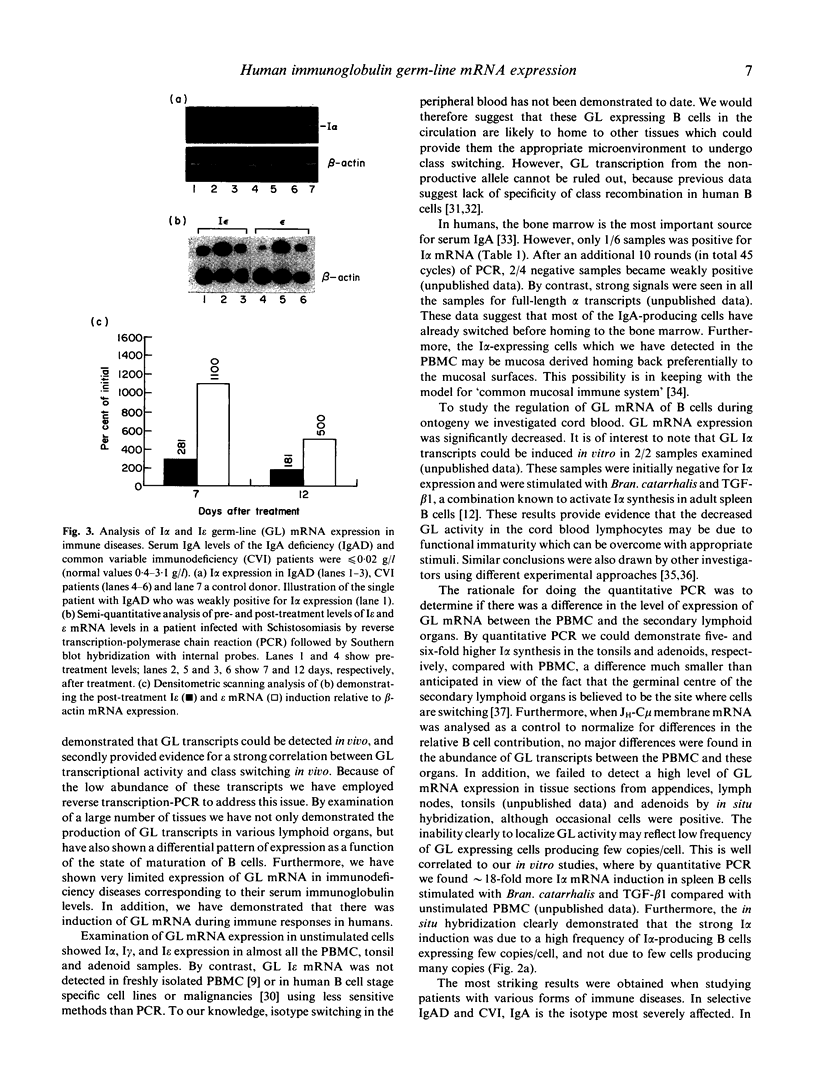


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alley C. D., Nash G. S., MacDermott R. P. Marked in vitro spontaneous secretion of IgA by human rib bone marrow mononuclear cells. J Immunol. 1982 Jun;128(6):2604–2608. [PubMed] [Google Scholar]
- Borzillo G. V., Cooper M. D., Kubagawa H., Landay A., Burrows P. D. Isotype switching in human B lymphocyte malignancies occurs by DNA deletion: evidence for nonspecific switch recombination. J Immunol. 1987 Aug 15;139(4):1326–1335. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Esser C., Radbruch A. Rapid induction of transcription of unrearranged S gamma 1 switch regions in activated murine B cells by interleukin 4. EMBO J. 1989 Feb;8(2):483–488. doi: 10.1002/j.1460-2075.1989.tb03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evengård B., Hammarström L., Smith C. I., Johansson S. G., Linder E. Subclass distribution and IgE responses after treatment in human schistosomiasis. Clin Exp Immunol. 1988 Sep;73(3):383–388. [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Lefranc M. P., Rabbitts T. H. Mechanisms of divergence and convergence of the human immunoglobulin alpha 1 and alpha 2 constant region gene sequences. Cell. 1984 Mar;36(3):681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982 Dec 23;300(5894):709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- Gauchat J. F., Lebman D. A., Coffman R. L., Gascan H., de Vries J. E. Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to IgE production. J Exp Med. 1990 Aug 1;172(2):463–473. doi: 10.1084/jem.172.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S., Fort P., Crawford D. H., Lefranc M. P., Lefranc G. Sequence of a human immunoglobulin gamma 3 heavy chain constant region gene: comparison with the other human C gamma genes. Nucleic Acids Res. 1986 Feb 25;14(4):1779–1789. doi: 10.1093/nar/14.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam K. B., Christensson B., Hammarström L., Smith C. I. Analysis of human IgA subclasses by in situ hybridization and combined in situ hybridization/immunohistochemistry. J Immunol Methods. 1992 Oct 2;154(2):163–172. doi: 10.1016/0022-1759(92)90188-y. [DOI] [PubMed] [Google Scholar]
- Islam K. B., Nilsson L., Sideras P., Hammarström L., Smith C. I. TGF-beta 1 induces germ-line transcripts of both IgA subclasses in human B lymphocytes. Int Immunol. 1991 Nov;3(11):1099–1106. doi: 10.1093/intimm/3.11.1099. [DOI] [PubMed] [Google Scholar]
- Jung S., Rajewsky K., Radbruch A. Shutdown of class switch recombination by deletion of a switch region control element. Science. 1993 Feb 12;259(5097):984–987. doi: 10.1126/science.8438159. [DOI] [PubMed] [Google Scholar]
- Kerr W. G., Burrows P. D. Stage-specific transcription of germline IgH C gamma and C alpha regions during human B cell differentiation. Int Immunol. 1991 Nov;3(11):1059–1065. doi: 10.1093/intimm/3.11.1059. [DOI] [PubMed] [Google Scholar]
- Kraal G., Weissman I. L., Butcher E. C. Germinal centre B cells: antigen specificity and changes in heavy chain class expression. Nature. 1982 Jul 22;298(5872):377–379. doi: 10.1038/298377a0. [DOI] [PubMed] [Google Scholar]
- Lebman D. A., Nomura D. Y., Coffman R. L., Lee F. D. Molecular characterization of germ-line immunoglobulin A transcripts produced during transforming growth factor type beta-induced isotype switching. Proc Natl Acad Sci U S A. 1990 May;87(10):3962–3966. doi: 10.1073/pnas.87.10.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon G. G., Perry R. P. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5'-nontranslatable exon. Nature. 1985 Dec 5;318(6045):475–478. doi: 10.1038/318475a0. [DOI] [PubMed] [Google Scholar]
- Li B., Sehajpal P. K., Khanna A., Vlassara H., Cerami A., Stenzel K. H., Suthanthiran M. Differential regulation of transforming growth factor beta and interleukin 2 genes in human T cells: demonstration by usage of novel competitor DNA constructs in the quantitative polymerase chain reaction. J Exp Med. 1991 Nov 1;174(5):1259–1262. doi: 10.1084/jem.174.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzker S., Alt F. W. Structure and expression of germ line immunoglobulin gamma 2b transcripts. Mol Cell Biol. 1988 Apr;8(4):1849–1852. doi: 10.1128/mcb.8.4.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Battey J., Ney R., Kirsch I. R., Leder P. Duplication and deletion in the human immunoglobulin epsilon genes. Cell. 1982 Jun;29(2):691–699. doi: 10.1016/0092-8674(82)90185-4. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Islam K. B., Olafsson O., Zalcberg I., Samakovlis C., Hammarström L., Smith C. I., Sideras P. Structure of TGF-beta 1-induced human immunoglobulin C alpha 1 and C alpha 2 germ-line transcripts. Int Immunol. 1991 Nov;3(11):1107–1115. doi: 10.1093/intimm/3.11.1107. [DOI] [PubMed] [Google Scholar]
- Olerup O., Smith C. I., Hammarström L. Different amino acids at position 57 of the HLA-DQ beta chain associated with susceptibility and resistance to IgA deficiency. Nature. 1990 Sep 20;347(6290):289–290. doi: 10.1038/347289a0. [DOI] [PubMed] [Google Scholar]
- Pastorelli G., Rousset F., Pène J., Peronne C., Roncarolo M. G., Tovo P. A., de Vries J. E. Cord blood B cells are mature in their capacity to switch to IgE-producing cells in response to interleukin-4 in vitro. Clin Exp Immunol. 1990 Oct;82(1):114–119. doi: 10.1111/j.1365-2249.1990.tb05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Milstein C. P. Human immunoglobulin heavy chain genes: evolutionary comparisons of C mu, C delta and C gamma genes and associated switch sequences. Nucleic Acids Res. 1981 Sep 25;9(18):4509–4524. doi: 10.1093/nar/9.18.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rothman P., Lutzker S., Cook W., Coffman R., Alt F. W. Mitogen plus interleukin 4 induction of C epsilon transcripts in B lymphoid cells. J Exp Med. 1988 Dec 1;168(6):2385–2389. doi: 10.1084/jem.168.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman P., Lutzker S., Gorham B., Stewart V., Coffman R., Alt F. W. Structure and expression of germline immunoglobulin gamma 3 heavy chain gene transcripts: implications for mitogen and lymphokine directed class-switching. Int Immunol. 1990;2(7):621–627. doi: 10.1093/intimm/2.7.621. [DOI] [PubMed] [Google Scholar]
- Schaffer F. M., Palermos J., Zhu Z. B., Barger B. O., Cooper M. D., Volanakis J. E. Individuals with IgA deficiency and common variable immunodeficiency share polymorphisms of major histocompatibility complex class III genes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8015–8019. doi: 10.1073/pnas.86.20.8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinson E., Fernandez C., Stavnezer J. Induction of germ-line immunoglobulin heavy chain transcripts by mitogens and interleukins prior to switch recombination. Eur J Immunol. 1990 May;20(5):1079–1084. doi: 10.1002/eji.1830200520. [DOI] [PubMed] [Google Scholar]
- Shapira S. K., Jabara H. H., Thienes C. P., Ahern D. J., Vercelli D., Gould H. J., Geha R. S. Deletional switch recombination occurs in interleukin-4-induced isotype switching to IgE expression by human B cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7528–7532. doi: 10.1073/pnas.88.17.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Mizuta T. R., Kanamori H., Suzuki N., Okamoto M., Kuze K., Ohno H., Doi S., Fukuhara S., Hassan M. S. Production of sterile transcripts of C gamma genes in an IgM-producing human neoplastic B cell line that switches to IgG-producing cells. Int Immunol. 1989;1(6):631–642. doi: 10.1093/intimm/1.6.631. [DOI] [PubMed] [Google Scholar]
- Sideras P., Nilsson L., Islam K. B., Ericsson H., Hammarström L., Smith C. I. Quantitative and qualitative analysis of human IgG subclass specific mRNA using solution hybridization. Scand J Immunol. 1991 Nov;34(5):557–564. doi: 10.1111/j.1365-3083.1991.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Sideras P., Nilsson L., Islam K. B., Quintana I. Z., Freihof L., Rosén A., Juliusson G., Hammarström L., Smith C. I. Transcription of unrearranged Ig H chain genes in human B cell malignancies. Biased expression of genes encoded within the first duplication unit of the Ig H chain locus. J Immunol. 1992 Jul 1;149(1):244–252. [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Tucci A., Mouzaki A., James H., Bonnefoy J. Y., Zubler R. H. Are cord blood B cells functionally mature? Clin Exp Immunol. 1991 Jun;84(3):389–394. [PMC free article] [PubMed] [Google Scholar]
- Vetrie D., Vorechovský I., Sideras P., Holland J., Davies A., Flinter F., Hammarström L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993 Jan 21;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Webb C. F., Cooper M. D., Burrows P. D., Griffin J. A. Immunoglobulin gene rearrangements and deletions in human Epstein-Barr virus-transformed cell lines producing different IgG and IgA subclasses. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5495–5499. doi: 10.1073/pnas.82.16.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein P. D., Cebra J. J. The preference for switching to IgA expression by Peyer's patch germinal center B cells is likely due to the intrinsic influence of their microenvironment. J Immunol. 1991 Dec 15;147(12):4126–4135. [PubMed] [Google Scholar]
- Xu L., Gorham B., Li S. C., Bottaro A., Alt F. W., Rothman P. Replacement of germ-line epsilon promoter by gene targeting alters control of immunoglobulin heavy chain class switching. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3705–3709. doi: 10.1073/pnas.90.8.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., DePinho R. A., Zimmerman K. A., Lutzker S. G., Rosenberg N., Alt F. W. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 1986 Dec 1;5(12):3259–3266. doi: 10.1002/j.1460-2075.1986.tb04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]





