Abstract
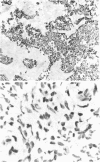
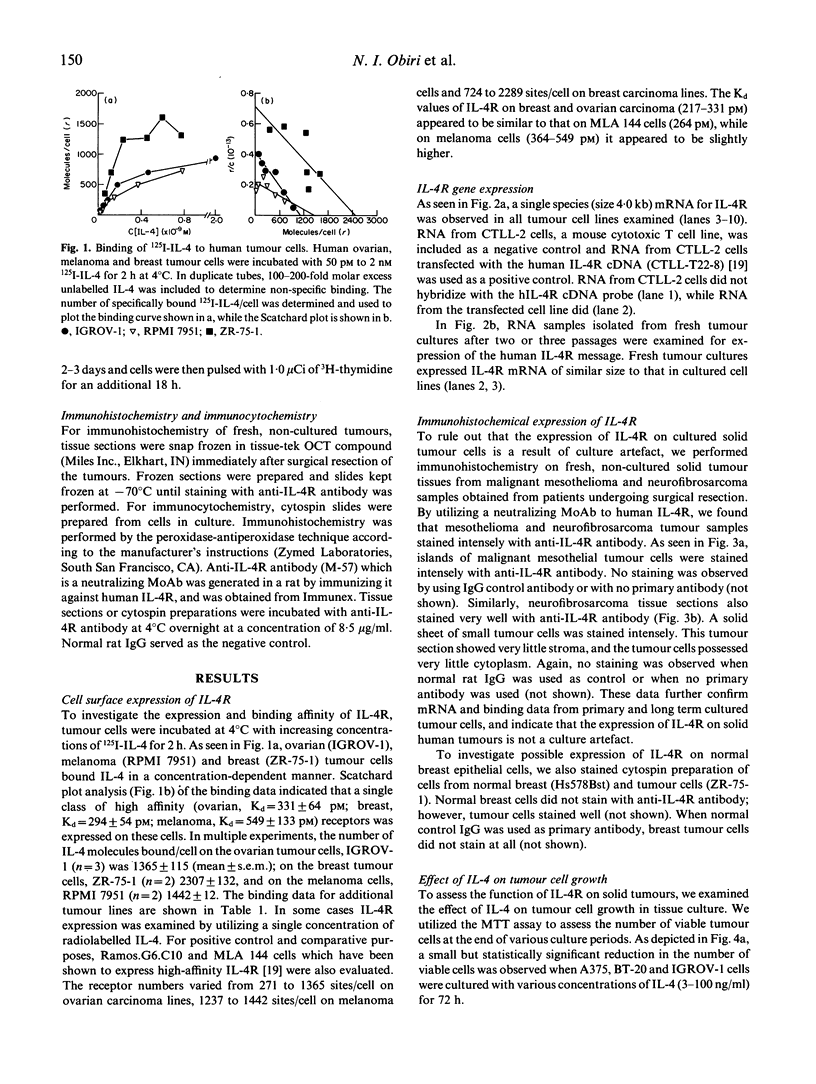
It has previously been shown that murine sarcoma cells express high-affinity IL-4 receptors (IL-4R) which are internalized after binding to the ligand (Puri et al., Cancer Res 1991; 51:3011-7). We have also reported that human renal cell carcinoma cells express high-affinity IL-4R, and IL-4 inhibits tumour growth in vitro (Obiri et al., J Clin Invest 1993; 91:88). In this study we investigated the expression and function of IL-4R on other human solid tumours. Human melanoma, ovarian carcinoma and breast carcinoma cell lines were assessed for the cell surface expression of IL-4R by radio-ligand receptor binding and for IL-4R gene expression by Northern blot analysis. Primary cultures of mesothelioma and neurofibrosarcoma cells were similarly investigated. Human melanoma, ovarian carcinoma and breast carcinoma cell lines expressed IL-4R on their cell surface with a dissociation constant (Kd) of 140-549 pM. These tumour lines expressed a single 4 kb species of mRNA for IL-4R. Similarly, primary cultures of mesothelioma and neurofibrosarcoma cells were positive for the IL-4R mRNA by Northern blot analysis. Fresh, non-cultured mesothelioma and neurofibrosarcoma tumour sections were also positive for the presence of IL-4R as determined by immunohistochemistry of frozen sections using anti-IL-4R antibody. In order to study possible functions of IL-4R, we evaluated the effects of IL-4 on cell growth and its effect on MHC antigen expression in the presence or absence of interferon-gamma (IFN-gamma). In tissue culture, IL-4 reduced the growth of tumour cell lines and primary cell cultures studied. IL-4 had very little effect on MHC class I antigen expression on ovarian, breast and melanoma cell lines; however, MHC class II (HLA-DR) expression was enhanced on melanoma and breast carcinoma cells. IL-4 also enhanced the IFN-gamma-induced class II expression on melanoma and breast carcinoma cells. Taken together, our observations indicate that IL-4R are expressed on a variety of human solid tumours and these receptors may be functional. IL-4 alone and in combination with IFN-gamma may play a role in host immune response against cancers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akashi K., Shibuya T., Harada M., Takamatsu Y., Uike N., Eto T., Niho Y. Interleukin 4 suppresses the spontaneous growth of chronic myelomonocytic leukemia cells. J Clin Invest. 1991 Jul;88(1):223–230. doi: 10.1172/JCI115281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Le Gros G., Conrad D. H., Finkelman F. D., Paul W. E. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990 Aug 15;145(4):1127–1136. [PubMed] [Google Scholar]
- Bogdan C., Stenger S., Röllinghoff M., Solbach W. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin 4 synergizes with interferon-gamma to activate murine macrophages for killing of Leishmania major amastigotes. Eur J Immunol. 1991 Feb;21(2):327–333. doi: 10.1002/eji.1830210213. [DOI] [PubMed] [Google Scholar]
- Bosco M., Giovarelli M., Forni M., Modesti A., Scarpa S., Masuelli L., Forni G. Low doses of IL-4 injected perilymphatically in tumor-bearing mice inhibit the growth of poorly and apparently nonimmunogenic tumors and induce a tumor-specific immune memory. J Immunol. 1990 Nov 1;145(9):3136–3143. [PubMed] [Google Scholar]
- Brown M., Hu-Li J., Paul W. E. IL-4/B cell stimulatory factor 1 stimulates T cell growth by an IL-2-independent mechanism. J Immunol. 1988 Jul 15;141(2):504–511. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- Foxwell B. M., Woerly G., Ryffel B. Identification of interleukin 4 receptor-associated proteins and expression of both high- and low-affinity binding on human lymphoid cells. Eur J Immunol. 1989 Sep;19(9):1637–1641. doi: 10.1002/eji.1830190918. [DOI] [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Park L. S., Morrissey P. J., Sassenfeld H., Price V., Urdal D. L., Widmer M. B. Regulation of murine T cell proliferation by B cell stimulatory factor-1. J Immunol. 1987 Aug 15;139(4):1148–1153. [PubMed] [Google Scholar]
- Green L. M., Reade J. L., Ware C. F. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods. 1984 May 25;70(2):257–268. doi: 10.1016/0022-1759(84)90190-x. [DOI] [PubMed] [Google Scholar]
- Gross N., Beck D., Favre S., Carrel S. In vitro antigenic modulation of human neuroblastoma cells induced by IFN-gamma, retinoic acid and dibutyryl cyclic AMP. Int J Cancer. 1987 Apr 15;39(4):521–529. doi: 10.1002/ijc.2910390420. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Kanakura Y., Fujita J., Takeda S., Nakano T., Tarui S., Honjo T., Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987 Jan 1;165(1):268–273. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon D. S., Banez M., Okun E., Morton D. L., Irie R. F. Modulation of human melanoma cells by interleukin-4 and in combination with gamma-interferon or alpha-tumor necrosis factor. Cancer Res. 1991 Apr 15;51(8):2002–2008. [PubMed] [Google Scholar]
- Hoon D. S., Okun E., Banez M., Irie R. F., Morton D. L. Interleukin 4 alone and with gamma-interferon or alpha-tumor necrosis factor inhibits cell growth and modulates cell surface antigens on human renal cell carcinomas. Cancer Res. 1991 Oct 15;51(20):5687–5693. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzerda R. L., March C. J., Mosley B., Lyman S. D., Vanden Bos T., Gimpel S. D., Din W. S., Grabstein K. H., Widmer M. B., Park L. S. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. J Exp Med. 1990 Mar 1;171(3):861–873. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Larche M., Lamb J. R., O'Hehir R. E., Imami-Shita N., Zanders E. D., Quint D. E., Moqbel R., Ritter M. A. Functional evidence for a monoclonal antibody that binds to the human IL-4 receptor. Immunology. 1988 Dec;65(4):617–622. [PMC free article] [PubMed] [Google Scholar]
- Larché M., Lamb J. R., Ritter M. A. A novel T-lymphocyte molecule that may function in the induction of self-tolerance and MHC-restriction within the human thymic microenvironment. Immunology. 1988 May;64(1):101–105. [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Swisher S. G., Minehart E. H., McBride W. H., Economou J. S. Interleukin-4 downregulates interleukin-6 production in human peripheral blood mononuclear cells. J Leukoc Biol. 1990 May;47(5):475–479. doi: 10.1002/jlb.47.5.475. [DOI] [PubMed] [Google Scholar]
- Lehn M., Kandil O., Arena C., Rein M. S., Remold H. G. Interleukin-4 fails to inhibit interferon-gamma-induced activation of human colostral macrophages. Cell Immunol. 1992 Apr 15;141(1):233–242. doi: 10.1016/0008-8749(92)90142-c. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A., Berenson J., Gera J. F., Waldburger K., Martinez-Maza O., Berek J. S. Resistance of human ovarian cancer cells to tumor necrosis factor and lymphokine-activated killer cells: correlation with expression of HER2/neu oncogenes. Cancer Res. 1990 Nov 15;50(22):7364–7370. [PubMed] [Google Scholar]
- Lowenthal J. W., Castle B. E., Christiansen J., Schreurs J., Rennick D., Arai N., Hoy P., Takebe Y., Howard M. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol. 1988 Jan 15;140(2):456–464. [PubMed] [Google Scholar]
- Milanese C., Richardson N. E., Reinherz E. L. Identification of a T helper cell-derived lymphokine that activates resting T lymphocytes. Science. 1986 Mar 7;231(4742):1118–1122. doi: 10.1126/science.2935936. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Obiri N. I., Hillman G. G., Haas G. P., Sud S., Puri R. K. Expression of high affinity interleukin-4 receptors on human renal cell carcinoma cells and inhibition of tumor cell growth in vitro by interleukin-4. J Clin Invest. 1993 Jan;91(1):88–93. doi: 10.1172/JCI116205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J. Interleukin-4: molecular structure and biochemical characteristics, biological function, and receptor expression. Year Immunol. 1989;5:126–159. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Grabstein K., Urdal D. L. Characterization of the high-affinity cell-surface receptor for murine B-cell-stimulating factor 1. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1669–1673. doi: 10.1073/pnas.84.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Sassenfeld H. M., Urdal D. L. Characterization of the human B cell stimulatory factor 1 receptor. J Exp Med. 1987 Aug 1;166(2):476–488. doi: 10.1084/jem.166.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. O., Everson M. P., Moldoveanu Z., Lue C., Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990 Sep 15;145(6):1740–1744. [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Puri R. K., Finbloom D. S., Leland P., Mostowski H., Siegel J. P. Expression of high-affinity IL-4 receptors on murine tumour infiltrating lymphocytes and their up-regulation by IL-2. Immunology. 1990 Aug;70(4):492–497. [PMC free article] [PubMed] [Google Scholar]
- Puri R. K., Ogata M., Leland P., Feldman G. M., FitzGerald D., Pastan I. Expression of high-affinity interleukin 4 receptors on murine sarcoma cells and receptor-mediated cytotoxicity of tumor cells to chimeric protein between interleukin 4 and Pseudomonas exotoxin. Cancer Res. 1991 Jun 1;51(11):3011–3017. [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Bergstedt-Lindqvist S., MacDonald H. R., Severinson E. Secretion of IgG1 induction factor by T cell clones and hybridomas. Eur J Immunol. 1985 Jun;15(6):586–593. doi: 10.1002/eji.1830150611. [DOI] [PubMed] [Google Scholar]
- Siegel J. P., Mostowski H. S. A bioassay for the measurement of human interleukin-4. J Immunol Methods. 1990 Sep 14;132(2):287–295. doi: 10.1016/0022-1759(90)90040-3. [DOI] [PubMed] [Google Scholar]
- Spits H., Yssel H., Paliard X., Kastelein R., Figdor C., de Vries J. E. IL-4 inhibits IL-2-mediated induction of human lymphokine-activated killer cells, but not the generation of antigen-specific cytotoxic T lymphocytes in mixed leukocyte cultures. J Immunol. 1988 Jul 1;141(1):29–36. [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Toi M., Bicknell R., Harris A. L. Inhibition of colon and breast carcinoma cell growth by interleukin-4. Cancer Res. 1992 Jan 15;52(2):275–279. [PubMed] [Google Scholar]
- Toi M., Harris A. L., Bicknell R. Interleukin-4 is a potent mitogen for capillary endothelium. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1287–1293. doi: 10.1016/0006-291x(91)91561-p. [DOI] [PubMed] [Google Scholar]
- Totpal K., Aggarwal B. B. Interleukin 4 potentiates the antiproliferative effects of tumor necrosis factor on various tumor cell lines. Cancer Res. 1991 Aug 15;51(16):4266–4270. [PubMed] [Google Scholar]
- Tungekar M. F., Turley H., Dunnill M. S., Gatter K. C., Ritter M. A., Harris A. L. Interleukin 4 receptor expression on human lung tumors and normal lung. Cancer Res. 1991 Jan 1;51(1):261–264. [PubMed] [Google Scholar]
- de Maagd R. A., MacKenzie W. A., Schuurman H. J., Ritter M. A., Price K. M., Broekhuizen R., Kater L. The human thymus microenvironment: heterogeneity detected by monoclonal anti-epithelial cell antibodies. Immunology. 1985 Apr;54(4):745–754. [PMC free article] [PubMed] [Google Scholar]