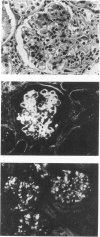
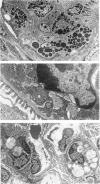
Abstract
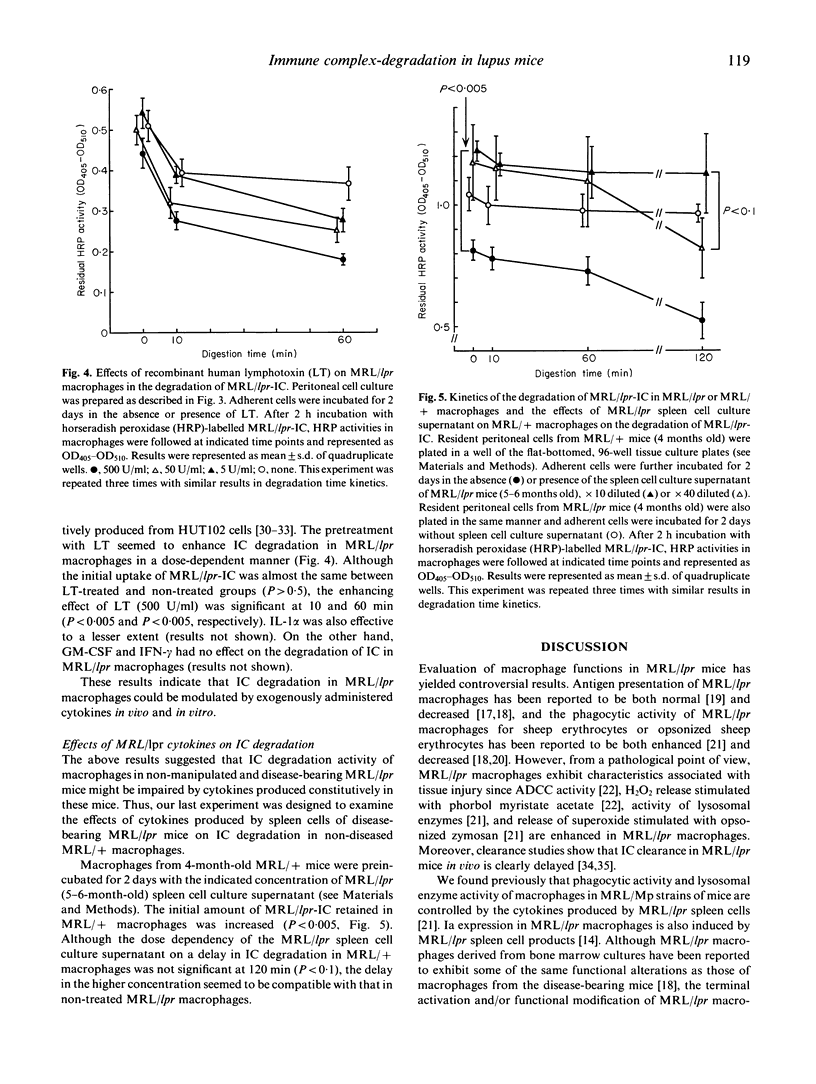
Impaired clearance of circulating and/or deposited immune complexes (IC) by the mononuclear phagocytic system is one of the major factors in the pathogenesis of IC diseases. MRL/Mp-lpr/lpr (MRL/lpr) lupus mice spontaneously develop a lethal glomerulonephritis associated with IC deposition. The ability of macrophages to degrade phagocytozed IC and regulation of this degradation in MRL/lpr mice were examined. In 4-month-old MRL/lpr mice, macrophages accumulated in the affected glomeruli and these macrophages contained many phagosomes containing electron-dense bodies. When culture supernatant of human T cell line HUT102 was administered intraperitoneally into disease-bearing MRL/lpr mice, degradation of these electron-dense bodies in the macrophages in glomeruli was noted. We developed a quantitative in vitro assay for IC degradation activity of MRL/lpr resident peritoneal macrophages (RPM) using peroxidase-labelled IC derived from MRL/lpr mouse sera. The ability of the RPM to degrade IC was remarkably enhanced by the pretreatment with HUT102 cell products and the related human recombinant cytokines, lymphotoxin and IL-1 alpha. Moreover, pretreatment of RPM from non-diseased MRL/Mp-+/+ mice with the culture supernatant of spleen cells from diseased MRL/lpr mice reduced their IC degradation activity. These results suggested that the ability of macrophages to degrade IC in MRL/Mp strains of mice is under the regulation of cytokines, and the impaired ability in the disease-bearing mice may be the result of abnormalities in the cytokine system in these mice.
Full text
PDF

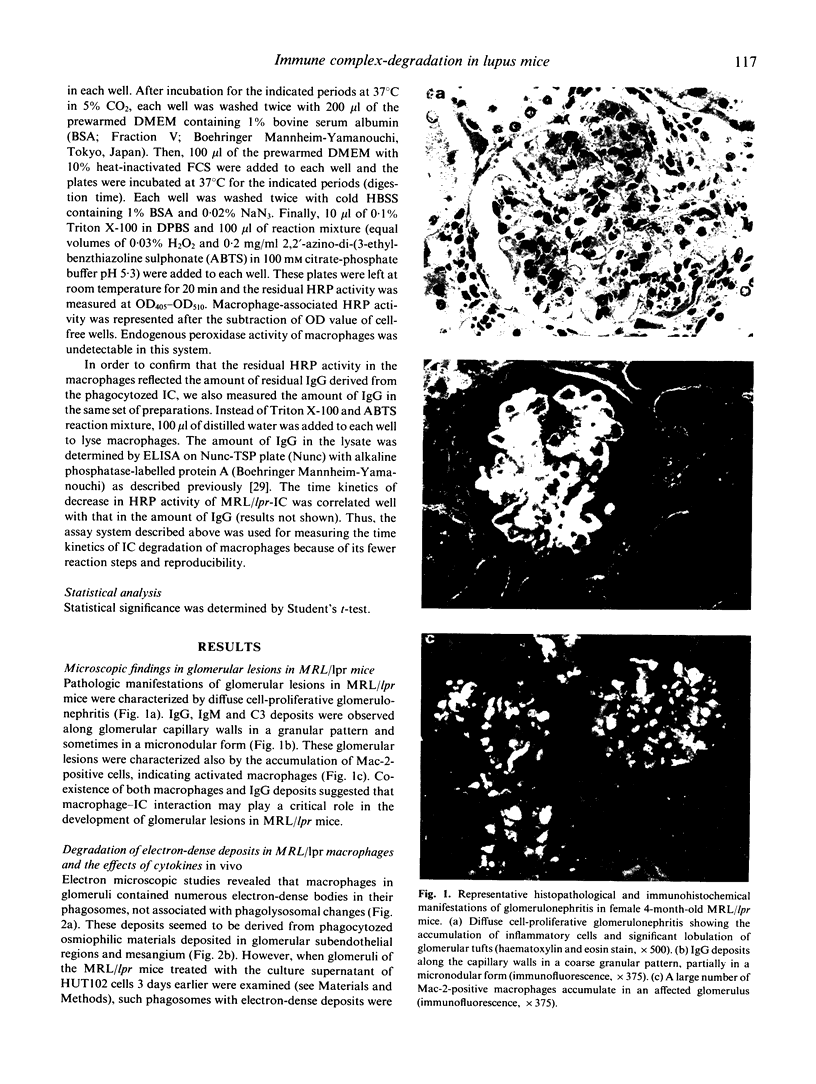
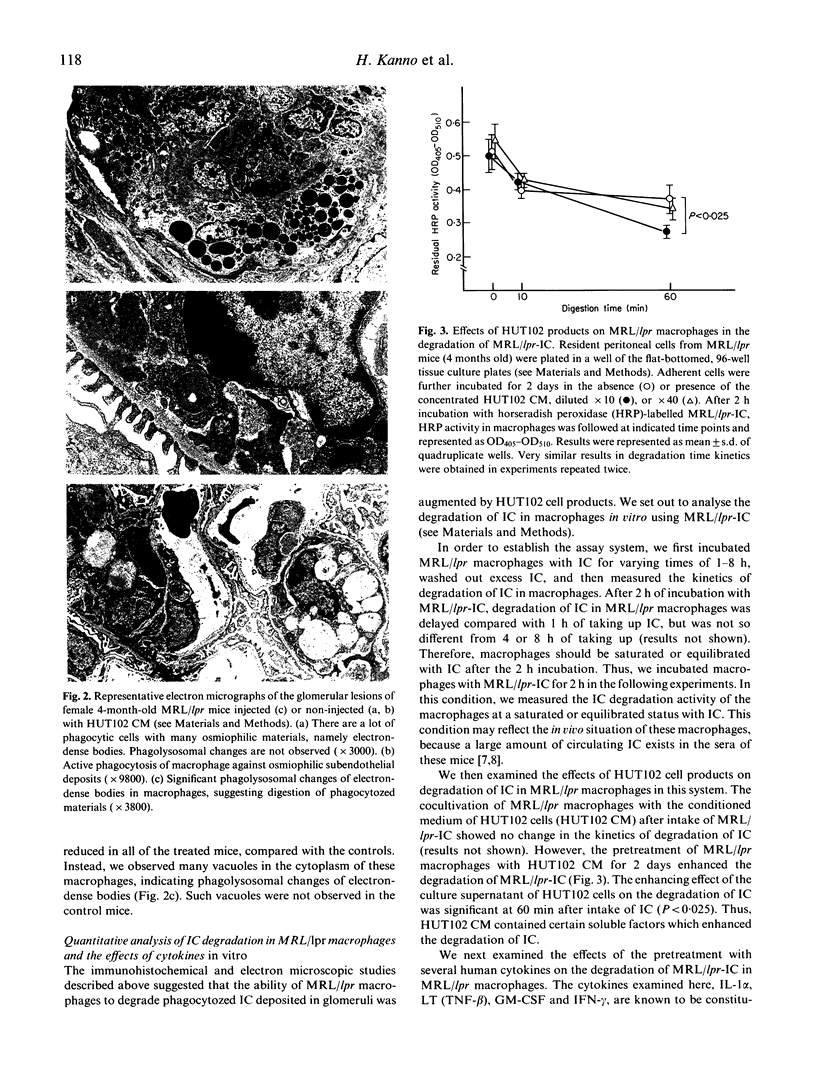


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M. A., Leslie R. G., Reeves W. G. The stimulation of superoxide anion production in guinea-pig peritoneal macrophages and neutrophils by phorbol myristate acetate, opsonized zymosan and IgG2-containing soluble immune complexes. Immunology. 1983 Apr;48(4):657–665. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Hill M., Haigh A. M., Stanley E. R. Stimulation of macrophage phagocytic but not bactericidal activity by colony-stimulating factor 1. Infect Immun. 1989 May;57(5):1512–1516. doi: 10.1128/iai.57.5.1512-1516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell P. A., Seehra M. S., Leslie R. G., Reeves W. G. Chemiluminescence of guinea pig peritoneal macrophages stimulated by immune precipitates and soluble immune complexes. Eur J Immunol. 1980 Dec;10(12):966–968. doi: 10.1002/eji.1830101214. [DOI] [PubMed] [Google Scholar]
- Dang-Vu A. P., Pisetsky D. S., Weinberg J. B. Functional alterations of macrophages in autoimmune MRL-lpr/lpr mice. J Immunol. 1987 Mar 15;138(6):1757–1761. [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Heyman B., Nose M., Weigle W. O. Carbohydrate chains on IgG2b: a requirement for efficient feedback immunosuppression. J Immunol. 1985 Jun;134(6):4018–4023. [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984 Jul;133(1):227–233. [PubMed] [Google Scholar]
- Jones F. S., Pisetsky D. S., Kurlander R. J. Defects in mononuclear phagocytic system (MPS) function in autoimmune MRL-lpr/lpr mice. Clin Immunol Immunopathol. 1985 Jul;36(1):30–39. doi: 10.1016/0090-1229(85)90036-4. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Roths J. B. Increase in macrophage Ia expression in autoimmune mice: role of the Ipr gene. J Immunol. 1982 Sep;129(3):923–925. [PubMed] [Google Scholar]
- Kobayashi M., Plunkett J. M., Masunaka I. K., Yamamoto R. S., Granger G. A. The human LT system. XII. Purification and functional studies of LT and "TNF-like" LT forms from a continuous human T cell line. J Immunol. 1986 Sep 15;137(6):1885–1892. [PubMed] [Google Scholar]
- Kofler R., Schreiber R. D., Dixon F. J., Theofilopoulos A. N. Macrophage I-A/I-E expression and macrophage-stimulating lymphokines in murine lupus. Cell Immunol. 1984 Aug;87(1):92–100. doi: 10.1016/0008-8749(84)90133-3. [DOI] [PubMed] [Google Scholar]
- Le J., Prensky W., Henriksen D., Vilcek J. Synthesis of alpha and gamma interferons by a human cutaneous lymphoma with helper T-cell phenotype. Cell Immunol. 1982 Sep 1;72(1):157–165. doi: 10.1016/0008-8749(82)90293-3. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Unanue E. R. Spontaneous T-cell lymphokine production and enhanced macrophage Ia expression and tumoricidal activity in MRL-lpr mice. Clin Immunol Immunopathol. 1982 Nov;25(2):213–222. doi: 10.1016/0090-1229(82)90184-2. [DOI] [PubMed] [Google Scholar]
- Magilavy D. B., Hundley T. R., Steinberg A. D., Katona I. M. Hepatic reticuloendothelial system activation in autoimmune mice: differences between (NZB X NZW)F1 and MRL-lpr/lpr strains. Clin Immunol Immunopathol. 1987 Mar;42(3):386–398. doi: 10.1016/0090-1229(87)90027-4. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Morrison A. D., Pruzanski W., Ranadive N. S. Release of lysosomal enzymes from human polymorphonuclear leukocytes by soluble intermediate immune complexes. Scand J Rheumatol. 1978;7(4):241–246. doi: 10.3109/03009747809095663. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Davidson W. F., Yetter R. A., Murphy E. D., Roths J. B., Coffman R. L. Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol. 1982 Dec;129(6):2612–2615. [PubMed] [Google Scholar]
- Mountz J. D., Huppi K. E., Seldin M. F., Mushinski J. F., Steinberg A. D. T cell receptor gene expression in autoimmune mice. J Immunol. 1986 Aug 1;137(3):1029–1036. [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Nakanishi A., Ashiba T., Imai Y., Osawa T. Functional defects of culture-grown bone marrow-derived macrophages from autoimmune MRL/MpJ-lpr/lpr mice. Microbiol Immunol. 1987;31(2):155–167. doi: 10.1111/j.1348-0421.1987.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Nemazee D. A., Studer S., Steinmetz M., Dembić Z., Kiefer M. The lymphoproliferating cells of MRL-lpr/lpr mice are a polyclonal population that bear the T lymphocyte receptor for antigen. Eur J Immunol. 1985 Aug;15(8):760–764. doi: 10.1002/eji.1830150804. [DOI] [PubMed] [Google Scholar]
- Nose M., Nishimura M., Kyogoku M. Analysis of granulomatous arteritis in MRL/Mp autoimmune disease mice bearing lymphoproliferative genes. The use of mouse genetics to dissociate the development of arteritis and glomerulonephritis. Am J Pathol. 1989 Aug;135(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca R., Wei F. Y., Singh P., Morasso M. I., Cantor H. Dysregulated expression of the T cell cytokine Eta-1 in CD4-8- lymphocytes during the development of murine autoimmune disease. J Exp Med. 1990 Oct 1;172(4):1177–1183. doi: 10.1084/jem.172.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. J., Steinberg A. D. Studies of peritoneal macrophage function in mice with systemic lupus erythematosus: depressed phagocytosis of opsonized sheep erythrocytes in vitro. Clin Immunol Immunopathol. 1983 Jun;27(3):387–402. doi: 10.1016/0090-1229(83)90091-0. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Lindner S. G., Gootenberg J., Popovic M., Hemmi H., Sarin P. S., Gallo R. C. Lymphokine production by cultured human T cells transformed by human T-cell leukemia-lymphoma virus-I. Science. 1984 Feb 17;223(4637):703–707. doi: 10.1126/science.6320367. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Jr, Tsurufuji M., Lu C. Y., Finberg R., Sy M. S. Comparison of antigen-specific T cell responses in autoimmune MRL/Mp-lpr/lpr and MRL/Mp-+/+ mice. J Immunol. 1984 Feb;132(2):633–639. [PubMed] [Google Scholar]
- Singh R. P., Patarca R., Schwartz J., Singh P., Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990 Jun 1;171(6):1931–1942. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Root R. K., Vaughan M. Phagocytosis in chronic granulomatous disease and the Chediak-Higashi syndrome. N Engl J Med. 1972 Jan 20;286(3):120–123. doi: 10.1056/NEJM197201202860302. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nose M., Sasaki J., Yamamoto T., Kyogoku M. IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J Immunol. 1991 Jul 15;147(2):515–519. [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Wofsy D., Hardy R. R., Seaman W. E. The proliferating cells in autoimmune MRL/lpr mice lack L3T4, an antigen on "helper" T cells that is involved in the response to class II major histocompatibility antigens. J Immunol. 1984 Jun;132(6):2686–2689. [PubMed] [Google Scholar]
- Yamashita U. Dysfunction of Ia-positive antigen-presenting cells in autoimmune mice. Cell Immunol. 1986 Feb;97(2):257–266. doi: 10.1016/0008-8749(86)90396-5. [DOI] [PubMed] [Google Scholar]
- Yamashita U., Shirakawa F., Nakamura H. Production of interleukin 1 by adult T cell leukemia (ATL) cell lines. J Immunol. 1987 May 15;138(10):3284–3289. [PubMed] [Google Scholar]
- Yui M. A., Brissette W. H., Brennan D. C., Wuthrich R. P., Rubin-Kelley V. E. Increased macrophage colony-stimulating factor in neonatal and adult autoimmune MRL-lpr mice. Am J Pathol. 1991 Aug;139(2):255–261. [PMC free article] [PubMed] [Google Scholar]