Abstract
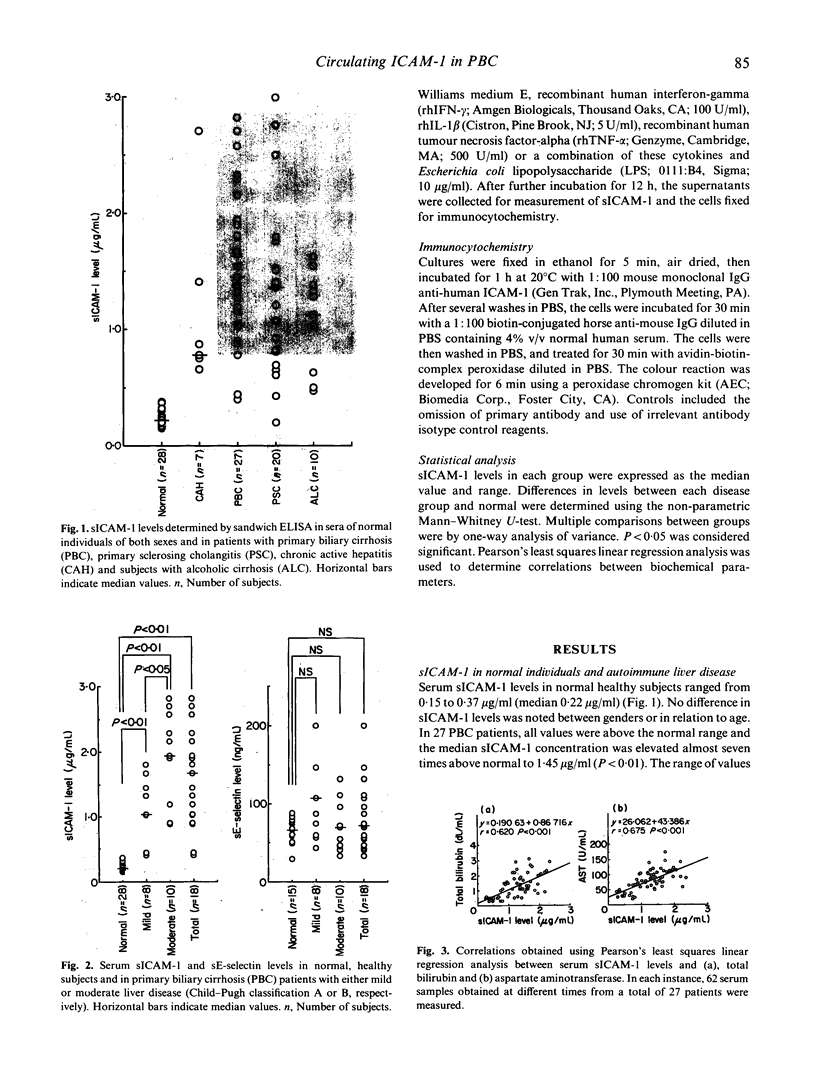
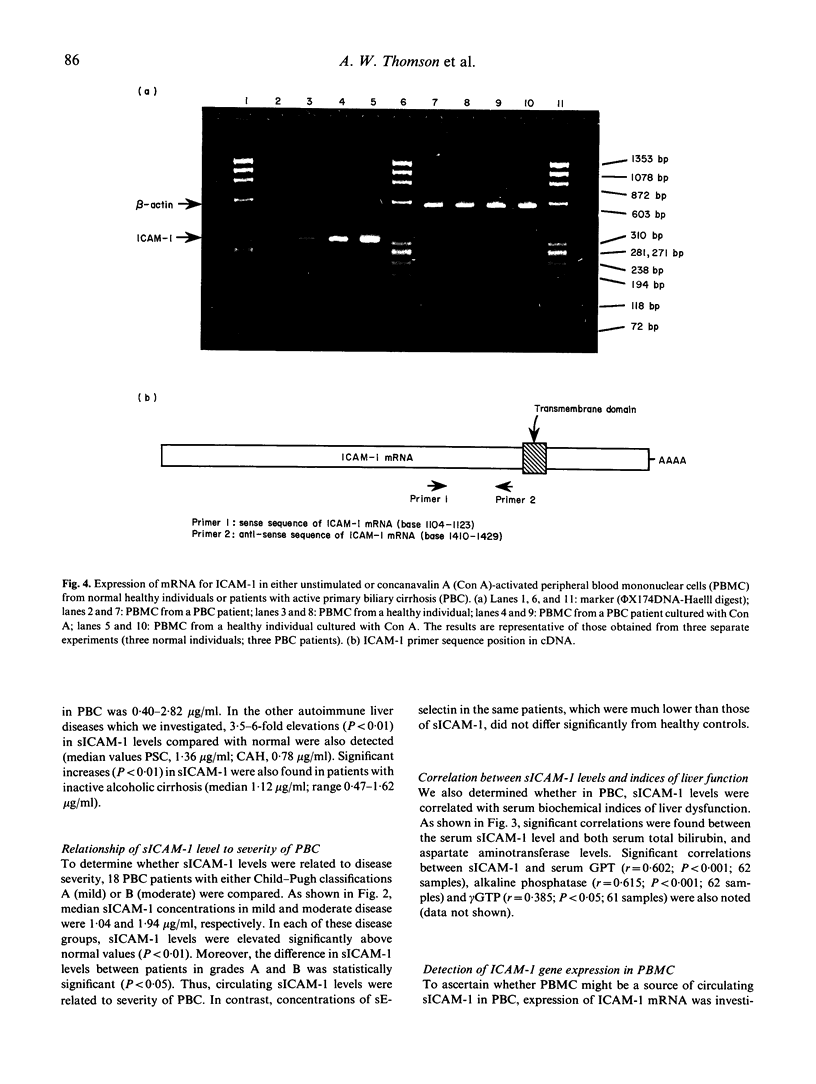
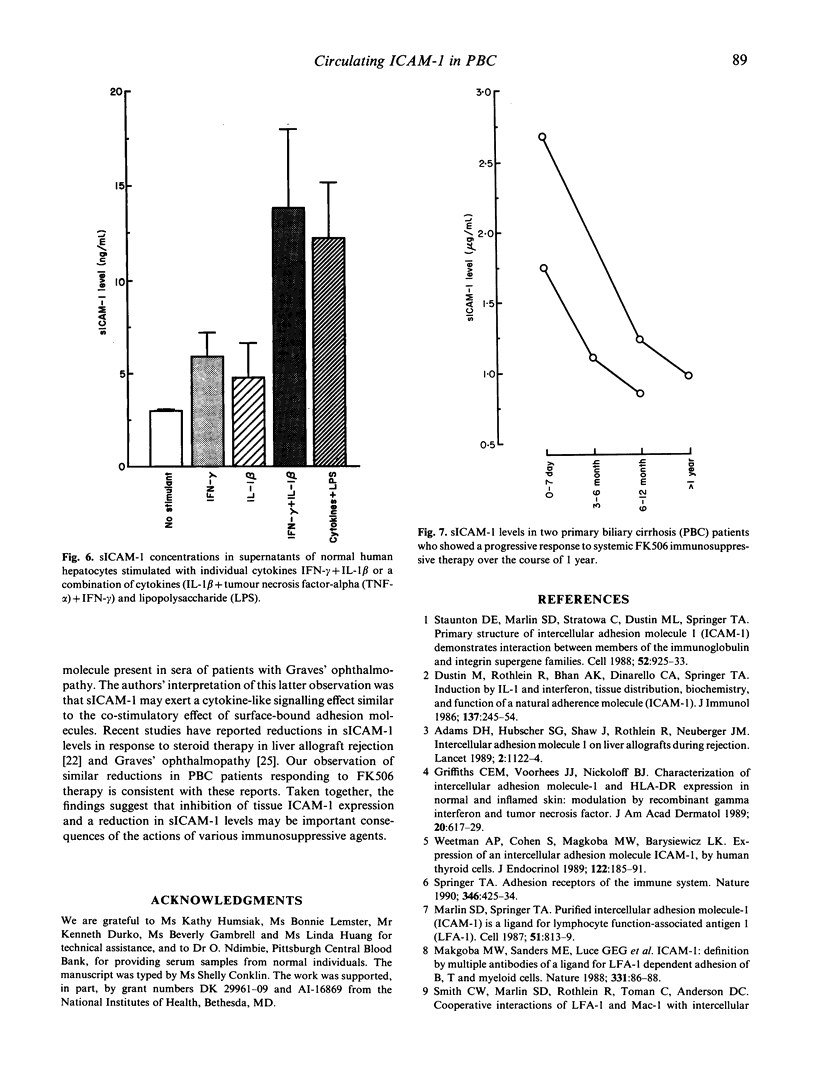
A circulating form of the membrane-bound ICAM-1 (CD54), a ligand for lymphocyte function-associated antigen-1 (LFA-1), has recently been identified in normal human serum. In this study, serum levels of soluble ICAM-1 (sICAM-1) were determined by sandwich ELISA both in normal healthy individuals of both sexes and in subjects with autoimmune liver diseases. Patients with primary biliary cirrhosis (PBC), primary sclerosing cholangitis and chronic active hepatitis (autoimmune) showed significant elevations in sICAM-1 compared with normal healthy subjects. The median level in PBC was approximately seven-fold above normal. Significant elevations in sICAM-1 were also detected, however, in patients with inactive alcoholic cirrhosis, suggesting that impaired liver clearance might at least in part account for the increased serum levels seen in patients with autoimmune liver disease. In patients with PBC, sICAM-1 levels were related to summary assessment of disease severity (Child-Pugh classification) and correlated significantly with serum biochemical indices of liver function, including measures both of cholestasis and liver cell injury. In contrast, serum levels of E-selectin did not differ significantly from healthy controls. Although it has been suggested that peripheral blood mononuclear cells (PBMC) may be a source of sICAM-1, investigation of ICAM-1 gene expression by reverse transcriptase polymerase chain reaction revealed similar basal levels of ICAM-1 message in PBMC of normal individuals and those with active PBC. This suggests that PBMC may not be a significant source of sICAM-1 in this disease. Similar increases in ICAM-1 mRNA expression were found in cultured, concanavalin A (Con A)-stimulated lymphocytes of both PBC patients and controls. Significantly, stimulation of cultured, normal human hepatocytes with proinflammatory cytokines and endotoxin induced cell surface expression of ICAM-1 and the secretion/shedding of sICAM-1 into the hepatocyte culture medium. This new finding suggests that hepatocytes may be an important source of sICAM-1 in autoimmune and other chronic liver diseases. The possible role of sICAM-1 in inflammatory disorders remains to be determined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Hubscher S. G., Shaw J., Johnson G. D., Babbs C., Rothlein R., Neuberger J. M. Increased expression of intercellular adhesion molecule 1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 1991 Sep;14(3):426–431. [PubMed] [Google Scholar]
- Adams D. H., Hubscher S. G., Shaw J., Rothlein R., Neuberger J. M. Intercellular adhesion molecule 1 on liver allografts during rejection. Lancet. 1989 Nov 11;2(8672):1122–1125. doi: 10.1016/s0140-6736(89)91489-x. [DOI] [PubMed] [Google Scholar]
- Adams D. H., Mainolfi E., Burra P., Neuberger J. M., Ayres R., Elias E., Rothlein R. Detection of circulating intercellular adhesion molecule-1 in chronic liver diseases. Hepatology. 1992 Sep;16(3):810–814. doi: 10.1002/hep.1840160330. [DOI] [PubMed] [Google Scholar]
- Adams D. H., Mainolfi E., Elias E., Neuberger J. M., Rothlein R. Detection of circulating intercellular adhesion molecule-1 after liver transplantation--evidence of local release within the liver during graft rejection. Transplantation. 1993 Jan;55(1):83–87. doi: 10.1097/00007890-199301000-00016. [DOI] [PubMed] [Google Scholar]
- Broomé U., Hultcrantz R., Scheynius A. Lack of concomitant expression of ICAM-1 and HLA-DR on bile duct cells from patients with primary sclerosing cholangitis and primary biliary cirrhosis. Scand J Gastroenterol. 1993 Feb;28(2):126–130. doi: 10.3109/00365529309096058. [DOI] [PubMed] [Google Scholar]
- Cosimi A. B., Conti D., Delmonico F. L., Preffer F. I., Wee S. L., Rothlein R., Faanes R., Colvin R. B. In vivo effects of monoclonal antibody to ICAM-1 (CD54) in nonhuman primates with renal allografts. J Immunol. 1990 Jun 15;144(12):4604–4612. [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Griffiths C. E., Voorhees J. J., Nickoloff B. J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989 Apr;20(4):617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Soluble intercellular adhesion molecule-1 (sICAM-1) in sera of patients with Graves' ophthalmopathy and thyroid diseases. Clin Exp Immunol. 1993 May;92(2):296–302. doi: 10.1111/j.1365-2249.1993.tb03395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kvale D., Brandtzaeg P. Immune modulation of adhesion molecules ICAM-1 (CD54) and LFA-3 (CD58) in human hepatocytic cell lines. J Hepatol. 1993 Mar;17(3):347–352. doi: 10.1016/s0168-8278(05)80216-8. [DOI] [PubMed] [Google Scholar]
- Lampeter E. R., Kishimoto T. K., Rothlein R., Mainolfi E. A., Bertrams J., Kolb H., Martin S. Elevated levels of circulating adhesion molecules in IDDM patients and in subjects at risk for IDDM. Diabetes. 1992 Dec;41(12):1668–1671. doi: 10.2337/diab.41.12.1668. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg J. F., Smeets E. F., Neefjes J. J., Shaffer M. A., Cinek T., Jeunhomme T. M., Ahern T. J., Buurman W. A. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992 Dec;77(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego H., Israel Y., Blake J. E., Medline A. Assessment of prognostic factors in alcoholic liver disease: toward a global quantitative expression of severity. Hepatology. 1983 Nov-Dec;3(6):896–905. doi: 10.1002/hep.1840030602. [DOI] [PubMed] [Google Scholar]
- Pugh R. N., Murray-Lyon I. M., Dawson J. L., Pietroni M. C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973 Aug;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Mainolfi E. A., Czajkowski M., Marlin S. D. A form of circulating ICAM-1 in human serum. J Immunol. 1991 Dec 1;147(11):3788–3793. [PubMed] [Google Scholar]
- Seth R., Raymond F. D., Makgoba M. W. Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet. 1991 Jul 13;338(8759):83–84. doi: 10.1016/0140-6736(91)90077-3. [DOI] [PubMed] [Google Scholar]
- Shijubo N., Imai K., Aoki S., Hirasawa M., Sugawara H., Koba H., Tsujisaki M., Sugiyama T., Hinoda Y., Yachi A. Circulating intercellular adhesion molecule-1 (ICAM-1) antigen in sera of patients with idiopathic pulmonary fibrosis. Clin Exp Immunol. 1992 Jul;89(1):58–62. doi: 10.1111/j.1365-2249.1992.tb06877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Carroll P. B., McCauley J., Woo J., Abu-Elmagd K., Starzl T. E., Van Thiel D. H. FK 506: a novel immunosuppressant for treatment of autoimmune disease. Rationale and preliminary clinical experience at the University of Pittsburgh. Springer Semin Immunopathol. 1993;14(4):323–344. doi: 10.1007/BF00192307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Hepatic expression of intercellular adhesion molecule-1 (ICAM-1) in viral hepatitis B. Hepatology. 1990 Jul;12(1):148–154. doi: 10.1002/hep.1840120123. [DOI] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology. 1990 Jul;12(1):59–65. doi: 10.1002/hep.1840120110. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Makgoba M. W., Borysiewicz L. K. Expression of an intercellular adhesion molecule, ICAM-1, by human thyroid cells. J Endocrinol. 1989 Jul;122(1):185–191. doi: 10.1677/joe.0.1220185. [DOI] [PubMed] [Google Scholar]