Abstract
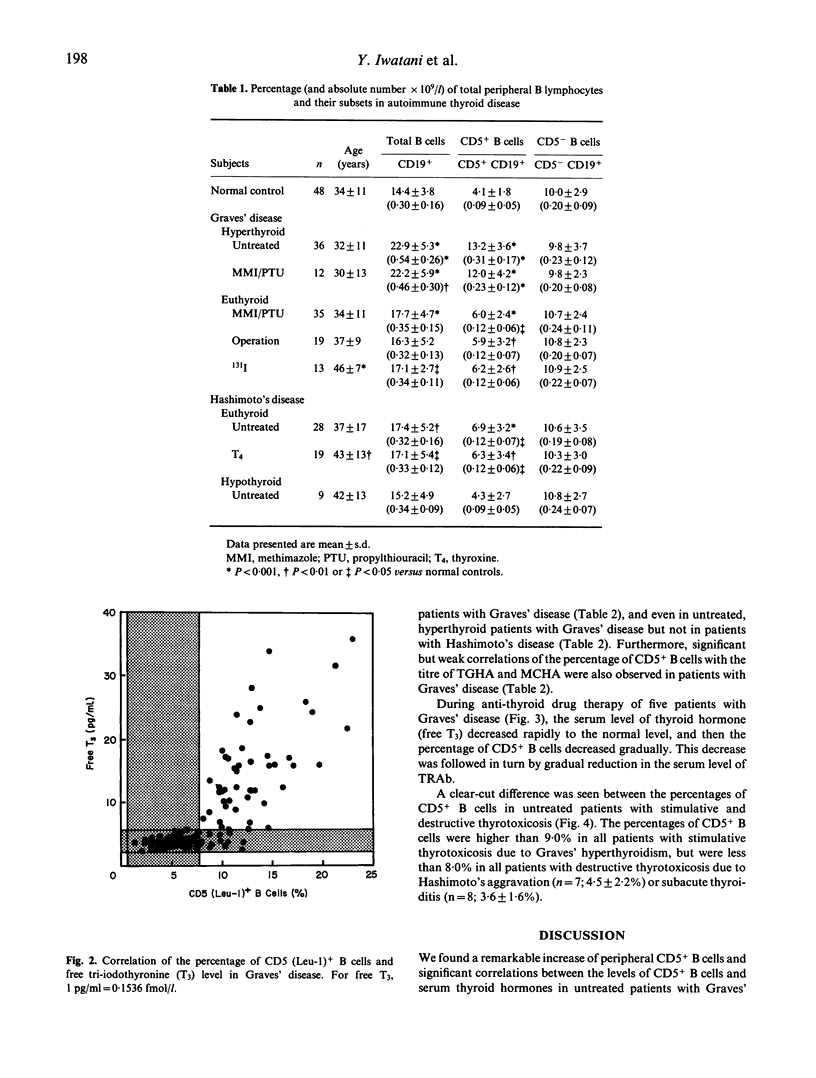
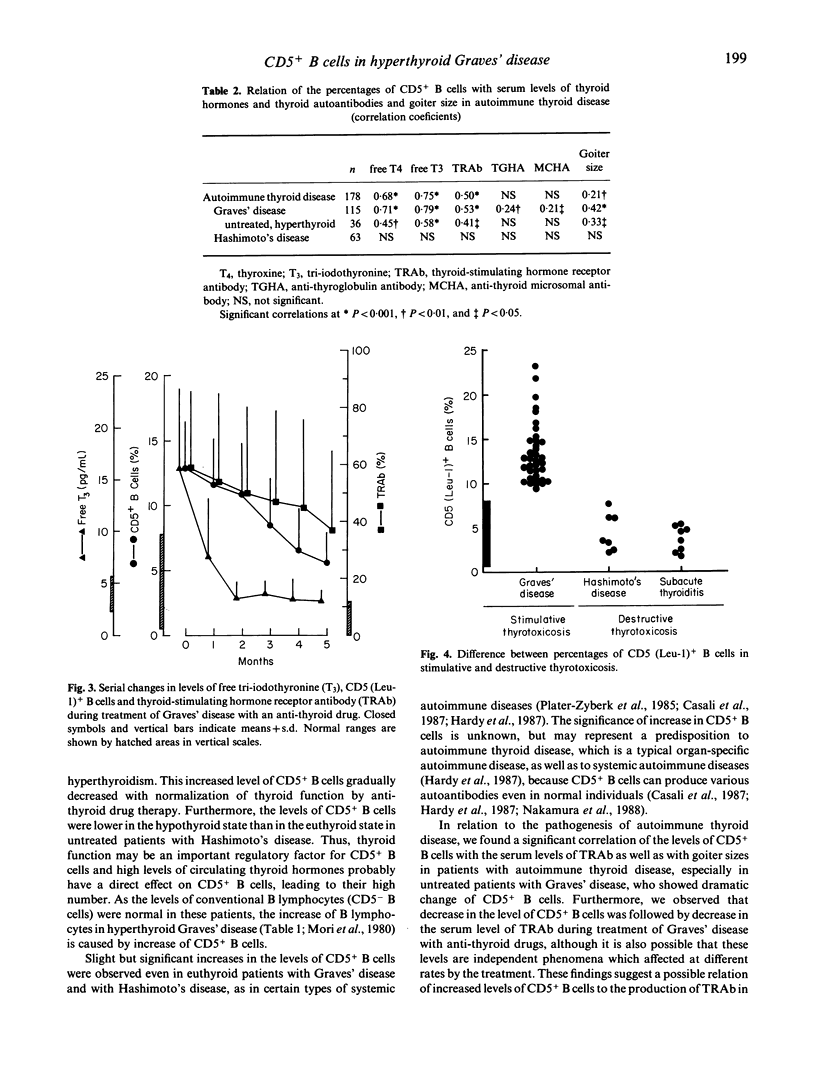
We examined the proportions of B lymphocytes bearing CD5 cell surface antigen (CD5+ B cells), which are capable of making autoantibodies, in peripheral blood from patients with various thyroid diseases. The level of CD5+ B cells was markedly increased (>9.0%) above the normal range (0.5-7.7%) in untreated, hyperthyroid patients with Graves' disease, although about 10% of the patients had no detectable serum thyroid-stimulating hormone (TSH) receptor antibody (TRAb). However, the levels of CD5+ B cells were normal in untreated patients with destructive thyrotoxicosis due to aggravation of Hashimoto's thyroiditis or subacute thyroiditis. In patients with stimulated hyperthyroid Graves' disease the levels of CD5+ B cells were correlated with those of thyroid hormones and TRAb, all significantly increased. However, once hyperthyroidism was controlled by anti-thyroid drugs, CD5+ B cells were decreased, followed in turn by reduction of TRAb. We conclude that the proportion of CD5+ B cells is useful as a therapeutic index and for diagnosis of Graves' disease and its differentiation from destruction-induced thyrotoxicosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amino N., Yabu Y., Miyai K., Fujie T., Azukizawa M., Onishi T., Kumahara Y. Differentiation of thyrotoxicosis induced by thyroid destruction from Graves' disease. Lancet. 1978 Aug 12;2(8085):344–346. doi: 10.1016/s0140-6736(78)92943-4. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Doniach D. Autoimmune thyroid disease. Annu Rev Med. 1986;37:353–359. doi: 10.1146/annurev.me.37.020186.002033. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Burastero S. E., Nakamura M., Inghirami G., Notkins A. L. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987 Apr 3;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- Gadol N., Ault K. A. Phenotypic and functional characterization of human Leu1 (CD5) B cells. Immunol Rev. 1986 Oct;93:23–34. doi: 10.1111/j.1600-065x.1986.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Gluck F. B., Nusynowitz M. L., Plymate S. Chronic lymphocytic thyroiditis, thyrotoxicosis, and low radioactive iodine uptake. Report of four cases. N Engl J Med. 1975 Sep 25;293(13):624–628. doi: 10.1056/NEJM197509252931302. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Iwatani Y., Amino N., Kabutomori O., Mori H., Tamaki H., Motoi S., Izumiguchi Y., Miyai K. Decrease of peripheral large granular lymphocytes in Graves' disease. Clin Exp Immunol. 1984 Jan;55(1):239–244. [PMC free article] [PubMed] [Google Scholar]
- Iwatani Y., Amino N., Mori H., Asari S., Izumiguchi Y., Kumahara Y., Miyai K. T lymphocyte subsets in autoimmune thyroid diseases and subacute thyroiditis detected with monoclonal antibodies. J Clin Endocrinol Metab. 1983 Feb;56(2):251–254. doi: 10.1210/jcem-56-2-251. [DOI] [PubMed] [Google Scholar]
- Lundberg J. G., Lewis W. M., Jr, Saunders J. F., 3rd, Mago-Leccia F. A major food web component in the orinoco river channel: evidence from planktivorous electric fishes. Science. 1987 Jul 3;237(4810):81–83. doi: 10.1126/science.237.4810.81. [DOI] [PubMed] [Google Scholar]
- McGregor A. M., Petersen M. M., McLachlan S. M., Rooke P., Smith B. R., Hall R. Carbimazole and the autoimmune response in Graves' disease. N Engl J Med. 1980 Aug 7;303(6):302–307. doi: 10.1056/NEJM198008073030603. [DOI] [PubMed] [Google Scholar]
- Meeker T. C., Miller R. A., Link M. P., Bindl J., Warnke R., Levy R. A unique human B lymphocyte antigen defined by a monoclonal antibody. Hybridoma. 1984 Winter;3(4):305–320. doi: 10.1089/hyb.1984.3.305. [DOI] [PubMed] [Google Scholar]
- Mori H., Amino N., Iwatani Y., Kabutomori O., Asari S., Motoi S., Miyai K., Kumahara Y. Increase of peripheral B lymphocytes in Graves' disease. Clin Exp Immunol. 1980 Oct;42(1):33–40. [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Burastero S. E., Notkins A. L., Casal P. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1)+ B cells are polyreactive. J Immunol. 1988 Jun 15;140(12):4180–4186. [PubMed] [Google Scholar]
- Okumura K., Hayakawa K., Tada T. Cell-to-cell interaction controlled by immunoglobulin genes. Role of Thy-1-, Lyt-1+, Ig+ (B') cell in allotype-restricted antibody production. J Exp Med. 1982 Aug 1;156(2):443–453. doi: 10.1084/jem.156.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C., Maini R. N., Lam K., Kennedy T. D., Janossy G. A rheumatoid arthritis B cell subset expresses a phenotype similar to that in chronic lymphocytic leukemia. Arthritis Rheum. 1985 Sep;28(9):971–976. doi: 10.1002/art.1780280903. [DOI] [PubMed] [Google Scholar]
- Renzi P., Ginns L. C. Analysis of T cell subsets in normal adults. Comparison of whole blood lysis technique to Ficoll-Hypaque separation by flow cytometry. J Immunol Methods. 1987 Apr 2;98(1):53–56. doi: 10.1016/0022-1759(87)90434-0. [DOI] [PubMed] [Google Scholar]
- Sherr D. H., Dorf M. E., Gibson M., Sidman C. L. Ly-1 B helper cells in autoimmune "viable motheaten" mice. J Immunol. 1987 Sep 15;139(6):1811–1817. [PubMed] [Google Scholar]
- Tötterman T. H., Karlsson F. A., Bengtsson M., Mendel-Hartvig I. Induction of circulating activated suppressor-like T cells by methimazole therapy for Graves' disease. N Engl J Med. 1987 Jan 1;316(1):15–22. doi: 10.1056/NEJM198701013160104. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M. Autoimmune thyroid disease: developments in our understanding. Endocr Rev. 1984 Spring;5(2):309–355. doi: 10.1210/edrv-5-2-309. [DOI] [PubMed] [Google Scholar]


