Abstract
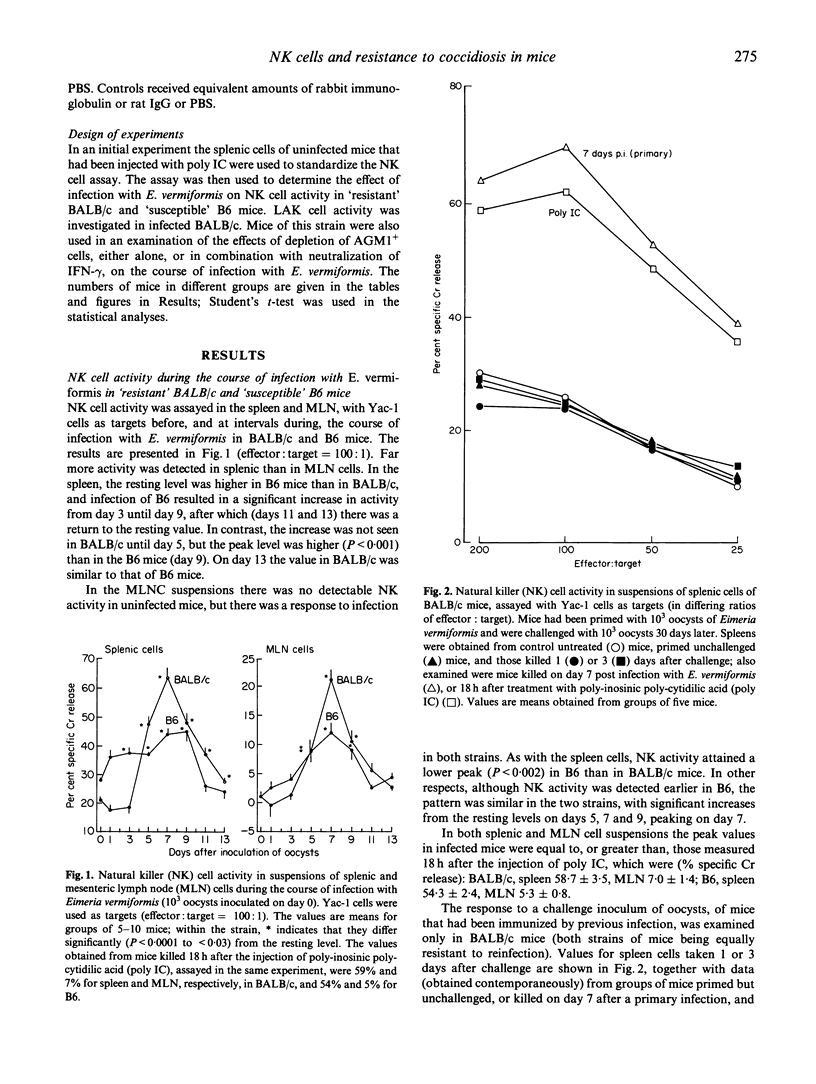
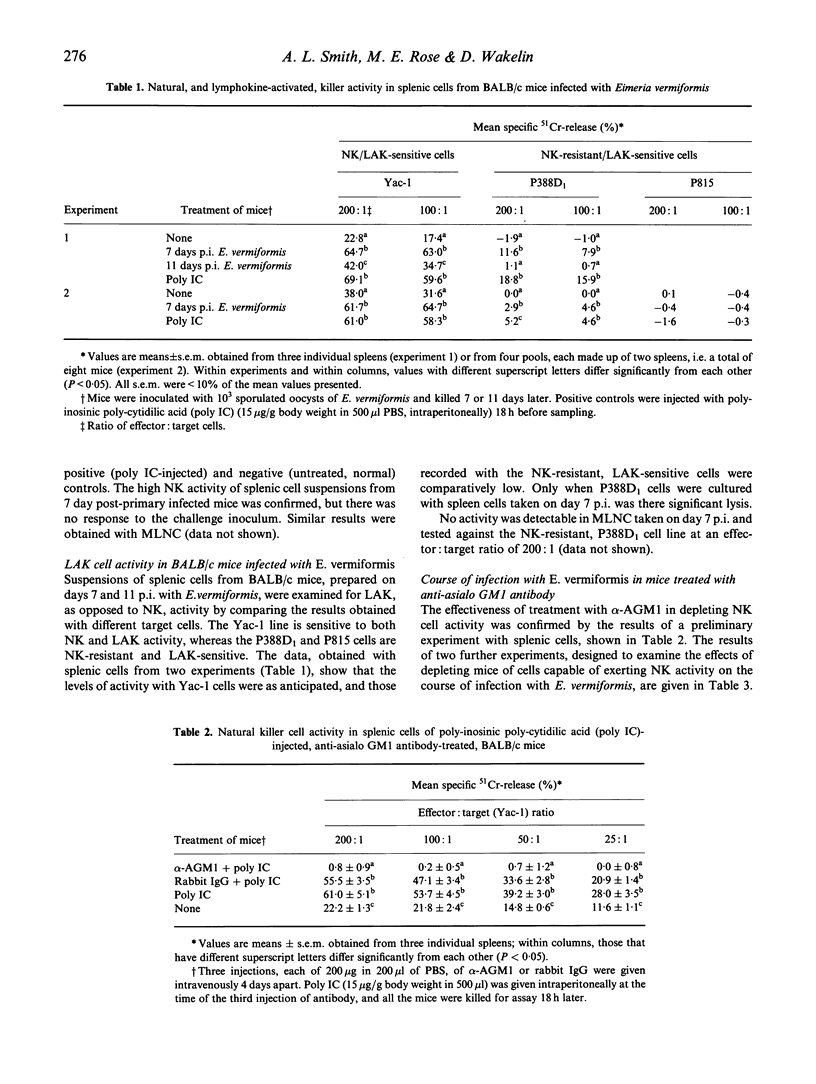
Natural killer (NK) activity, detected by the lysis of Yac-1 target cells, was examined in splenic and mesenteric lymph node (MLN) cells throughout the course of infection with Eimeria vermiformis in BALB/c and C57B1/6 (B6) mice. These strains are, respectively, relatively resistant and susceptible to primary infections, which render them equally, and completely, resistant to challenge. Resting levels of NK activity were higher in B6 than in BALB/c, and B6 responded earlier in the course of infection than BALB/c, but splenic peak values were higher in BALB/c; the pattern of response in MLN cells was similar in both strains, but the peak was higher in BALB/c. At the time (7 days p.i.) of peak NK response in BALB/c mice there was, depending upon the choice of NK-resistant/lymphokine-activated killer (LAK)-sensitive target cells, either little (P388D1), or no (P815) splenic LAK activity. Challenge of immunized BALB/c mice did not evoke a detectable NK response. Although the higher NK activity in BALB/c mice correlated with greater control of primary infection, depletion of NK activity (demonstrated in splenic cells) in vivo by treatment with anti-asialo GM1 antibodies did not greatly affect the course of infection. Furthermore, this treatment did not augment the exacerbation of infection produced by treatment with anti-interferon-gamma (IFN-gamma) MoAb, indicating that, at least in this system, NK cells are not a fundamentally important source of this controlling cytokine of eimerian infections. The results suggest that NK cells may not greatly influence the outcome of coccidial infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Huang K. Y., Albright J. F. Natural killer activity in mice infected with Trypanosoma musculi. Infect Immun. 1983 Jun;40(3):869–875. doi: 10.1128/iai.40.3.869-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993 Aug;5(4):503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- Chai J. Y., Lillehoj H. S. Isolation and functional characterization of chicken intestinal intra-epithelial lymphocytes showing natural killer cell activity against tumour target cells. Immunology. 1988 Jan;63(1):111–117. [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez B., Nicolás R., Galdeano A., Cisterna R., Cañavate M. L. Kinetics and regulation of NK activity by interleukin-2 and interferon in acute toxoplasmosis. Scand J Immunol. 1991 Nov;34(5):673–677. doi: 10.1111/j.1365-3083.1991.tb01591.x. [DOI] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Greenfield R. A., Abrams V. L., Crawford D. L., Kuhls T. L. Effect of abrogation of natural killer cell activity on the course of candidiasis induced by intraperitoneal administration and gastrointestinal candidiasis in mice with severe combined immunodeficiency. Infect Immun. 1993 Jun;61(6):2520–2525. doi: 10.1128/iai.61.6.2520-2525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H. P., Kasper L. H., Little J., Dubey J. P. Absence of a role for natural killer cells in the control of acute infection by Toxoplasma gondii oocysts. Clin Exp Immunol. 1988 Jun;72(3):394–399. [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Bancroft G. J. Leishmania donovani infection in scid mice: lack of tissue response and in vivo macrophage activation correlates with failure to trigger natural killer cell-derived gamma interferon production in vitro. Infect Immun. 1992 Oct;60(10):4335–4342. doi: 10.1128/iai.60.10.4335-4342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Splenic natural killer-cell activity in mice infected with Leishmania donovani. Cell Immunol. 1984 Apr 15;85(1):201–214. doi: 10.1016/0008-8749(84)90290-9. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Hinds S. E. Strain-dependent differences in murine susceptibility to coccidia. Infect Immun. 1979 Dec;26(3):1111–1115. doi: 10.1128/iai.26.3.1111-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H. S. Intestinal intraepithelial and splenic natural killer cell responses to eimerian infections in inbred chickens. Infect Immun. 1989 Jul;57(7):1879–1884. doi: 10.1128/iai.57.7.1879-1884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrt J. L., Shi Y. F. Murine major histocompatibility complex and immune response to Eimeria falciformis. Infect Immun. 1988 Jan;56(1):270–271. doi: 10.1128/iai.56.1.270-271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr E. L., Parr M. B., Zheng L. M., Young J. D. Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol Reprod. 1991 May;44(5):834–841. doi: 10.1095/biolreprod44.5.834. [DOI] [PubMed] [Google Scholar]
- Rohlman V. C., Kuhls T. L., Mosier D. A., Crawford D. L., Greenfield R. A. Cryptosporidium parvum infection after abrogation of natural killer cell activity in normal and severe combined immunodeficiency mice. J Parasitol. 1993 Apr;79(2):295–297. [PubMed] [Google Scholar]
- Rose M. E., Millard B. J. Eimeria vermiformis: host strains and the developmental cycle. Exp Parasitol. 1985 Dec;60(3):285–293. doi: 10.1016/0014-4894(85)90033-5. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Owen D. G., Hesketh P. Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology. 1984 Feb;88(Pt 1):45–54. doi: 10.1017/s0031182000054330. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Wakelin D., Hesketh P. Eimeria vermiformis: differences in the course of primary infection can be correlated with lymphocyte responsiveness in the BALB/c and C57BL/6 mouse, Mus musculus. Exp Parasitol. 1990 Oct;71(3):276–283. doi: 10.1016/0014-4894(90)90032-8. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Wakelin D., Hesketh P. Gamma interferon controls Eimeria vermiformis primary infection in BALB/c mice. Infect Immun. 1989 May;57(5):1599–1603. doi: 10.1128/iai.57.5.1599-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Wakelin D., Hesketh P. Interferon-gamma-mediated effects upon immunity to coccidial infections in the mouse. Parasite Immunol. 1991 Jan;13(1):63–74. doi: 10.1111/j.1365-3024.1991.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Scharton T. M., Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993 Aug 1;178(2):567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Oswald I. P., Hieny S., Gazzinelli R. T. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol. 1993 May 1;150(9):3982–3989. [PubMed] [Google Scholar]
- Skamene E., Stevenson M. M., Lemieux S. Murine malaria: dissociation of natural killer (NK) cell activity and resistance to Plasmodium chabaudi. Parasite Immunol. 1983 Nov;5(6):557–565. doi: 10.1111/j.1365-3024.1983.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Stockdale P. G., Stockdale M. J., Rickard M. D., Mitchell G. F. Mouse strain variation and effects of oocyst dose in infection of mice with Eimeria falciformis, a coccidian parasite of the large intestine. Int J Parasitol. 1985 Aug;15(4):447–452. doi: 10.1016/0020-7519(85)90032-3. [DOI] [PubMed] [Google Scholar]
- Subauste C. S., Dawson L., Remington J. S. Human lymphokine-activated killer cells are cytotoxic against cells infected with Toxoplasma gondii. J Exp Med. 1992 Dec 1;176(6):1511–1519. doi: 10.1084/jem.176.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar B. L., Kao T. C., Burris J. A., Finkelman F. D. Cryptosporidium infection in an adult mouse model. Independent roles for IFN-gamma and CD4+ T lymphocytes in protective immunity. J Immunol. 1991 Aug 1;147(3):1014–1022. [PubMed] [Google Scholar]
- Wakelin D., Rose M. E., Hesketh P., Else K. J., Grencis R. K. Immunity to coccidiosis: genetic influences on lymphocyte and cytokine responses to infection with Eimeria vermiformis in inbred mice. Parasite Immunol. 1993 Jan;15(1):11–19. doi: 10.1111/j.1365-3024.1993.tb00567.x. [DOI] [PubMed] [Google Scholar]


