Abstract
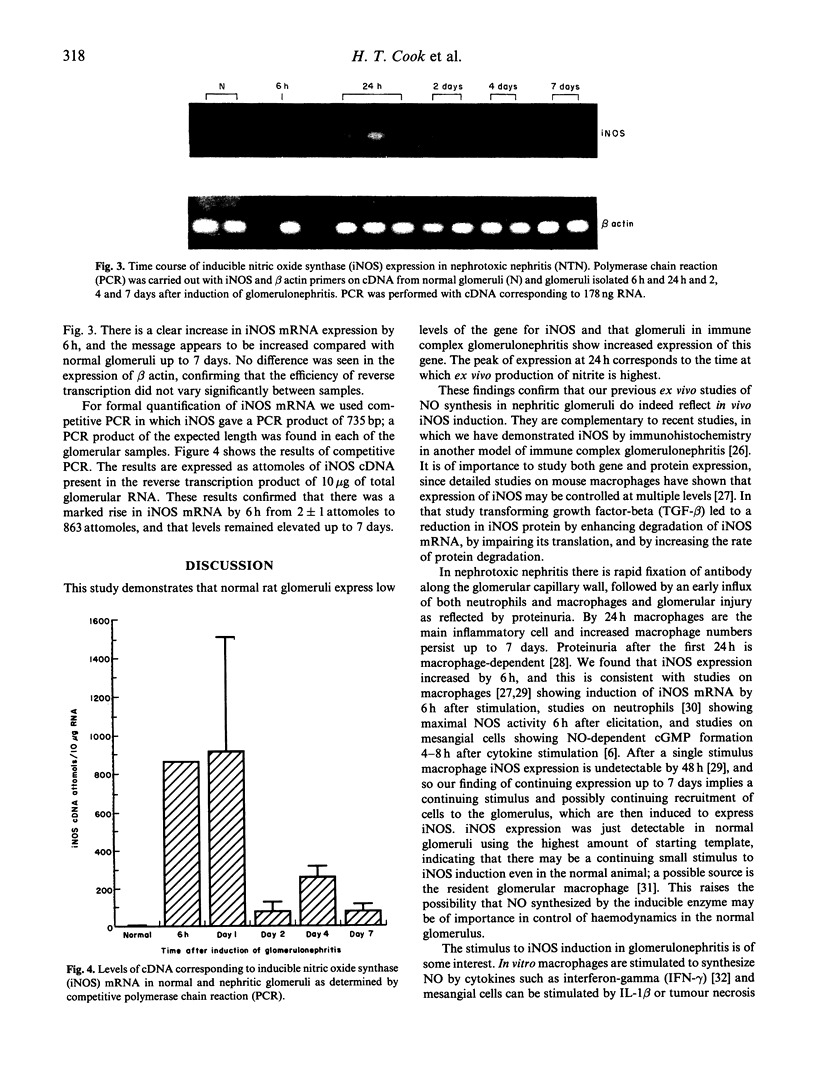
Nitrite, a stable product of nitric oxide (NO), is synthesized in vitro by glomeruli in experimental glomerulonephritis. We have now studied the expression of the gene for inducible NO synthase (iNOS) in accelerated nephrotoxic nephritis (NTN). The purpose of the study was to confirm in vivo induction of iNOS in this model of immune complex disease, and to relate the onset of induction and the level of expression to pathogenic events in the model. Glomeruli from rats with NTN were isolated at 6 h, 24 h and 2, 4 and 7 days and total RNA extracted. RNA (10 micrograms) was reverse transcribed and polymerase chain reaction (PCR) was performed with primers homologous to rat vascular smooth muscle iNOS and rat beta actin. A 222-base PCR product corresponding to iNOS mRNA was present in all experimental animals. iNOS expression was also found in activated macrophages, neutrophils and IL-1-stimulated but not unstimulated mesangial cells. Quantitative competitive PCR was carried out on glomerular samples using a 514-bp mutant of a 735-bp PCR product. iNOS expression was present at low levels in normal glomeruli and was markedly enhanced at 6 h after the induction of glomerulonephritis and peaked at 24 h. Increased iNOS expression persisted to day 7. beta actin mRNA levels were similar in all glomerular specimens. This study demonstrates that there is in vivo induction of iNOS in immune complex glomerulonephritis, corresponding to the generation of nitrite we have previously reported. iNOS gene expression is detectable within 6 h of induction of NTN, indicating the onset of gene transcription is closely related to the initial formation of immune complexes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Cattell V., Cook T., Moncada S. Glomeruli synthesize nitrite in experimental nephrotoxic nephritis. Kidney Int. 1990 Dec;38(6):1056–1060. doi: 10.1038/ki.1990.312. [DOI] [PubMed] [Google Scholar]
- Cattell V., Lianos E., Largen P., Cook T. Glomerular NO synthase activity in mesangial cell immune injury. Exp Nephrol. 1993 Jan-Feb;1(1):36–40. [PubMed] [Google Scholar]
- Cook H. T., Sullivan R. Glomerular nitrite synthesis in in situ immune complex glomerulonephritis in the rat. Am J Pathol. 1991 Nov;139(5):1047–1052. [PMC free article] [PubMed] [Google Scholar]
- Cunha F. Q., Assreuy J., Moncada S., Liew F. Y. Phagocytosis and induction of nitric oxide synthase in murine macrophages. Immunology. 1993 Jul;79(3):408–411. [PMC free article] [PubMed] [Google Scholar]
- Cunha F. Q., Assreuy J., Xu D., Charles I., Liew F. Y., Moncada S. Repeated induction of nitric oxide synthase and leishmanicidal activity in murine macrophages. Eur J Immunol. 1993 Jun;23(6):1385–1388. doi: 10.1002/eji.1830230631. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Jansen A., Cook T., Taylor G. M., Largen P., Riveros-Moreno V., Moncada S., Cattell V. Induction of nitric oxide synthase in rat immune complex glomerulonephritis. Kidney Int. 1994 Apr;45(4):1215–1219. doi: 10.1038/ki.1994.161. [DOI] [PubMed] [Google Scholar]
- Kleemann R., Rothe H., Kolb-Bachofen V., Xie Q. W., Nathan C., Martin S., Kolb H. Transcription and translation of inducible nitric oxide synthase in the pancreas of prediabetic BB rats. FEBS Lett. 1993 Aug 9;328(1-2):9–12. doi: 10.1016/0014-5793(93)80954-s. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Zheng Y. M., Heber-Katz E., Fraser N., Rorke L., Fu Z. F., Hanlon C., Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H., Harada N., Watanabe M., Yoshihara H., Katsuki Y., Shiga T. Synergistic stimulation of nitric oxide hemoglobin production in rats by recombinant interleukin 1 and tumor necrosis factor. Biochem Biophys Res Commun. 1992 Nov 30;189(1):392–397. doi: 10.1016/0006-291x(92)91571-7. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Langrehr J. M., Bergonia H. A., Murase N., Simmons R. L., Hoffman R. A. EPR detection of heme and nonheme iron-containing protein nitrosylation by nitric oxide during rejection of rat heart allograft. J Biol Chem. 1992 Jun 5;267(16):10994–10998. [PubMed] [Google Scholar]
- Lin R. F., Lin T. S., Tilton R. G., Cross A. H. Nitric oxide localized to spinal cords of mice with experimental allergic encephalomyelitis: an electron paramagnetic resonance study. J Exp Med. 1993 Aug 1;178(2):643–648. doi: 10.1084/jem.178.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markewitz B. A., Michael J. R., Kohan D. E. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest. 1993 May;91(5):2138–2143. doi: 10.1172/JCI116439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T. B., Palmer R. M., Moncada S. Induction of nitric oxide synthase in rat peritoneal neutrophils and its inhibition by dexamethasone. Eur J Immunol. 1991 Oct;21(10):2523–2527. doi: 10.1002/eji.1830211032. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Allen J. B., Mizel D. E., Albina J. E., Xie Q. W., Nathan C. F., Wahl S. M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993 Aug 1;178(2):749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Johnson K. J., Todd R. F., 3rd, Issekutz T. B., Miyasaka M., Tamatani T., Smith C. W., Anderson D. C., Ward P. A. Requirements for leukocyte adhesion molecules in nephrotoxic nephritis. J Clin Invest. 1993 Feb;91(2):577–587. doi: 10.1172/JCI116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nicolson A. G., Haites N. E., McKay N. G., Wilson H. M., MacLeod A. M., Benjamin N. Induction of nitric oxide synthase in human mesangial cells. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1269–1274. doi: 10.1006/bbrc.1993.1762. [DOI] [PubMed] [Google Scholar]
- Nunokawa Y., Ishida N., Tanaka S. Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Feb 26;191(1):89–94. doi: 10.1006/bbrc.1993.1188. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peten E. P., Garcia-Perez A., Terada Y., Woodrow D., Martin B. M., Striker G. E., Striker L. J. Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol. 1992 Nov;263(5 Pt 2):F951–F957. doi: 10.1152/ajprenal.1992.263.5.F951. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Rob P., Mülsch A., Fandrey J., Vosbeck K., Busse R. Interleukin 1 beta and tumour necrosis factor alpha induce a macrophage-type of nitric oxide synthase in rat renal mesangial cells. Eur J Biochem. 1992 Jan 15;203(1-2):251–255. doi: 10.1111/j.1432-1033.1992.tb19854.x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Schwarzenbach H. Interleukin 1 and tumor necrosis factor stimulate cGMP formation in rat renal mesangial cells. FEBS Lett. 1990 Oct 29;273(1-2):185–187. doi: 10.1016/0014-5793(90)81080-8. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Kiely J. M., Cotran R. S., Unanue E. R. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest. 1981 Oct;68(4):920–931. doi: 10.1172/JCI110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. A., Laubach V. E., Reep B. R., Wood E. R. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993 Nov 2;32(43):11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y., Bogdan C., Paik J., Xie Q. W., Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993 Aug 1;178(2):605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. R., Berger H., Jr, Sherman P. A., Lapetina E. G. Hepatocytes and macrophages express an identical cytokine inducible nitric oxide synthase gene. Biochem Biophys Res Commun. 1993 Mar 31;191(3):767–774. doi: 10.1006/bbrc.1993.1283. [DOI] [PubMed] [Google Scholar]





