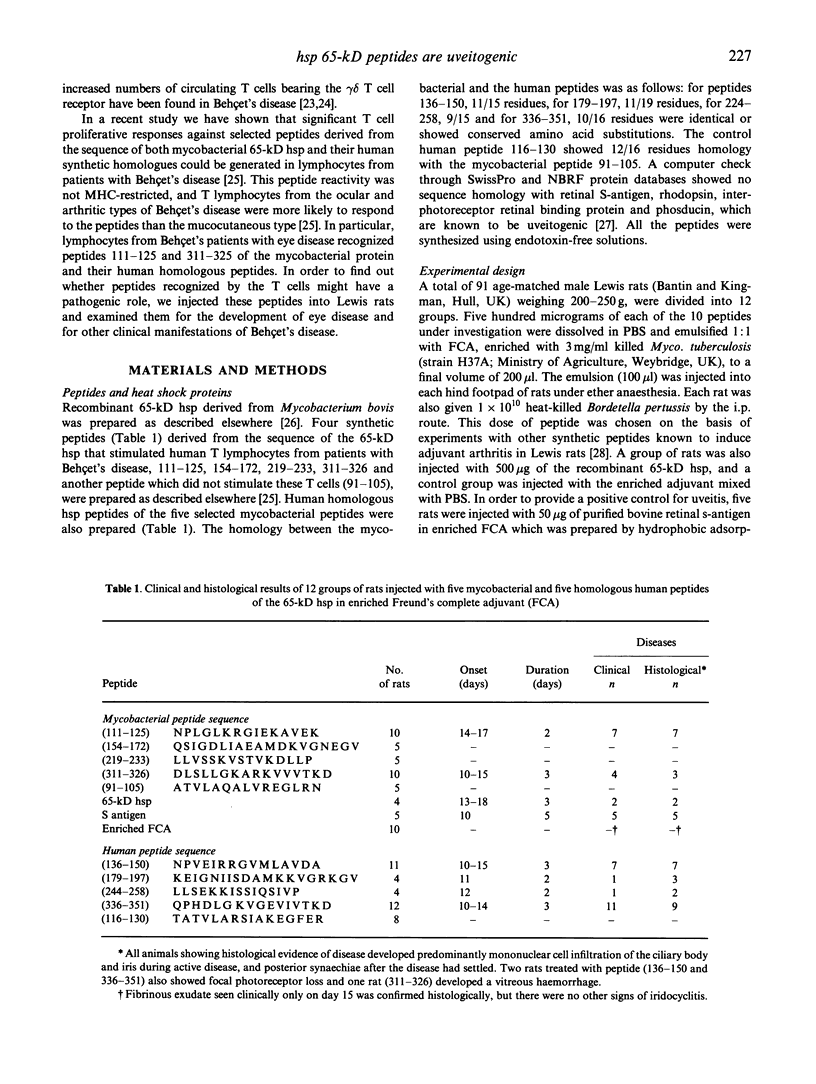
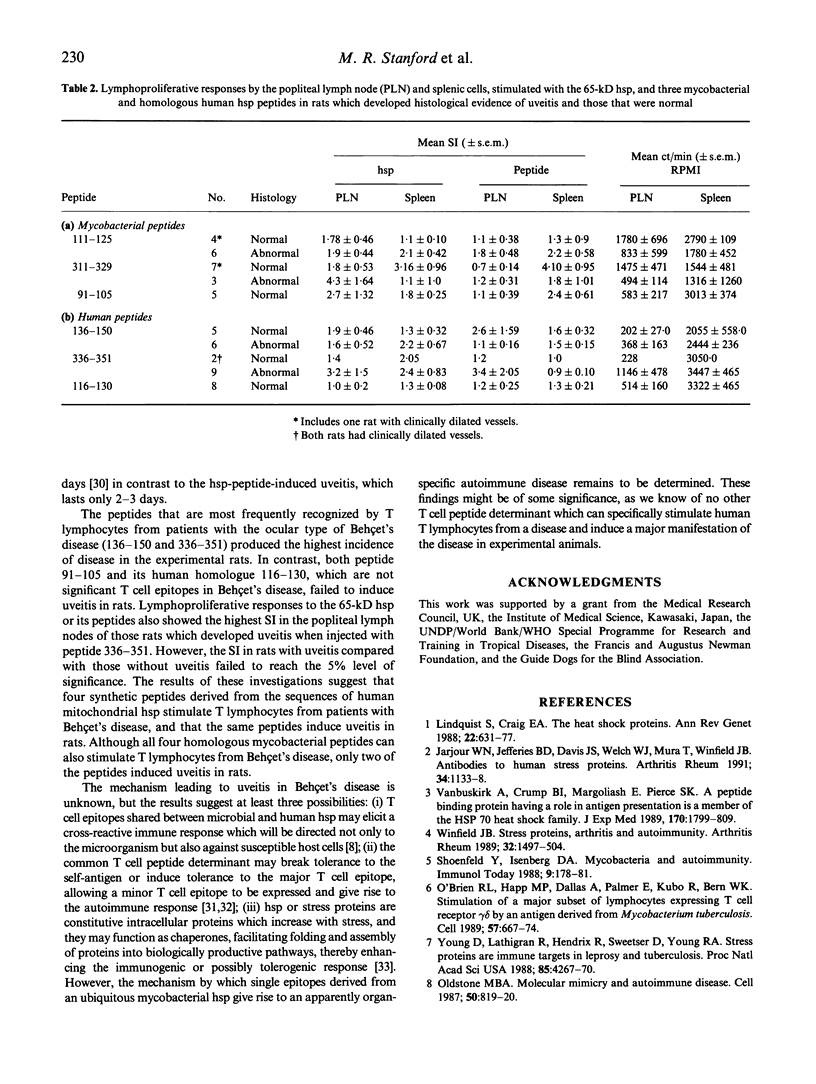
Abstract
Mycobacterial and homologous human heat shock protein T cell peptide epitopes specific for T lymphocytes in Behçet's disease were investigated for their pathogenicity in Lewis rats. The potential pathogenicity of eight peptides and two controls was assessed by administering the peptides in enriched Freund's adjuvant into the footpads of male Lewis rats. Anterior uveitis which is a major manifestation of Behçet's disease was induced with two out of the four mycobacterial and all four homologous human peptides. The most effective peptides inducing iridocyclitis in 64-75% of rats were peptides with amino acids 336-351 and 136-150, derived from the sequence of the human 60-kD heat shock protein. A few of the rats also showed evidence of focal loss of photoreceptors. These results suggest that selected peptides within heat shock protein 60 kD which function as T cell epitopes in Behçet's disease are capable of inducing uveitis in rats. This supports the view that the peptide T cell determinants may be involved in the pathogenesis of Behçet's disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander-Miller M. A., Burke K., Koszinowski U. H., Hansen T. H., Connolly J. M. Alloreactive cytotoxic T lymphocytes generated in the presence of viral-derived peptides show exquisite peptide and MHC specificity. J Immunol. 1993 Jul 1;151(1):1–10. [PubMed] [Google Scholar]
- Billingham M. E., Carney S., Butler R., Colston M. J. A mycobacterial 65-kD heat shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990 Jan 1;171(1):339–344. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Reshef T., Birk O. S., van der Zee R., Walker M. D., Cohen I. R. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon G., Sercarz E. How some T cells escape tolerance induction. Nature. 1989 Nov 9;342(6246):183–185. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Jenner P. J., Colston M. J., Bacon P. A. Recognition of a mycobacteria-specific epitope in the 65-kD heat-shock protein by synovial fluid-derived T cell clones. J Exp Med. 1990 Mar 1;171(3):831–841. doi: 10.1084/jem.171.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986 Aug 9;2(8502):305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour W. N., Jeffries B. D., Davis J. S., 4th, Welch W. J., Mimura T., Winfield J. B. Autoantibodies to human stress proteins. A survey of various rheumatic and other inflammatory diseases. Arthritis Rheum. 1991 Sep;34(9):1133–1138. doi: 10.1002/art.1780340909. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Duff G. Is there no role for heat-shock protein in diabetes? Lancet. 1991 Jan 12;337(8733):115–115. doi: 10.1016/0140-6736(91)90774-j. [DOI] [PubMed] [Google Scholar]
- Kaneko F., Takahashi Y., Muramatsu Y., Miura Y. Immunological studies on aphthous ulcer and erythema nodosum-like eruptions in Behcet's disease. Br J Dermatol. 1985 Sep;113(3):303–312. doi: 10.1111/j.1365-2133.1985.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Kasp E., Banga J. P., Brown E. C., Wicking J. M., Suleyman S., Ellis B. A., Sanders M. D., Dumonde D. C. An improved method for the purification of retinal S-antigen using selective hydrophobic adsorption chromatography. J Immunol Methods. 1987 Jun 26;100(1-2):147–152. doi: 10.1016/0022-1759(87)90183-9. [DOI] [PubMed] [Google Scholar]
- Lehner T., Lavery E., Smith R., van der Zee R., Mizushima Y., Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behçet's syndrome. Infect Immun. 1991 Apr;59(4):1434–1441. doi: 10.1128/iai.59.4.1434-1441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Petty R. E., Johnston W., McCormick A. Q., Hunt D. W., Rootman J., Rollins D. F. Uveitis and arthritis induced by adjuvant: clinical, immunologic and histologic characteristics. J Rheumatol. 1989 Apr;16(4):499–505. [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Ria F., Chan B. M., Scherer M. T., Smith J. A., Gefter M. L. Immunological activity of covalently linked T-cell epitopes. Nature. 1990 Jan 25;343(6256):381–383. doi: 10.1038/343381a0. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A. Mycobacteria and autoimmunity. Immunol Today. 1988 Jun;9(6):178–182. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- Skin hypersensitivity to streptococcal antigens and the induction of systemic symptoms by the antigens in Behçet's disease--a multicenter study. The Behcet's Disease Research Committee of Japan. J Rheumatol. 1989 Apr;16(4):506–511. [PubMed] [Google Scholar]
- Suzuki Y., Hoshi K., Matsuda T., Mizushima Y. Increased peripheral blood gamma delta+ T cells and natural killer cells in Behçet's disease. J Rheumatol. 1992 Apr;19(4):588–592. [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbuskirk A., Crump B. L., Margoliash E., Pierce S. K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989 Dec 1;170(6):1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B. Are heat-shock proteins relevant to the pathogenesis of inflammatory arthritis? Clin Exp Rheumatol. 1991 Jan-Feb;9(1):67–71. [PubMed] [Google Scholar]
- Winfield J. B. Stress proteins, arthritis, and autoimmunity. Arthritis Rheum. 1989 Dec;32(12):1497–1504. doi: 10.1002/anr.1780321202. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Hogervorst E. J., Hensen E. J., van der Zee R., van Embden J. D., Cohen I. R. A cartilage-mimicking T-cell epitope on a 65K mycobacterial heat-shock protein: adjuvant arthritis as a model for human rheumatoid arthritis. Curr Top Microbiol Immunol. 1989;145:27–43. doi: 10.1007/978-3-642-74594-2_3. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van den Broek M. F., Hogervorst E. J., Van Bruggen M. C., Van Eden W., van der Zee R., van den Berg W. B. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989 Aug 1;170(2):449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]