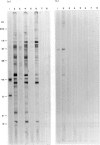
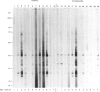
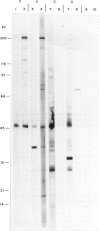
Abstract
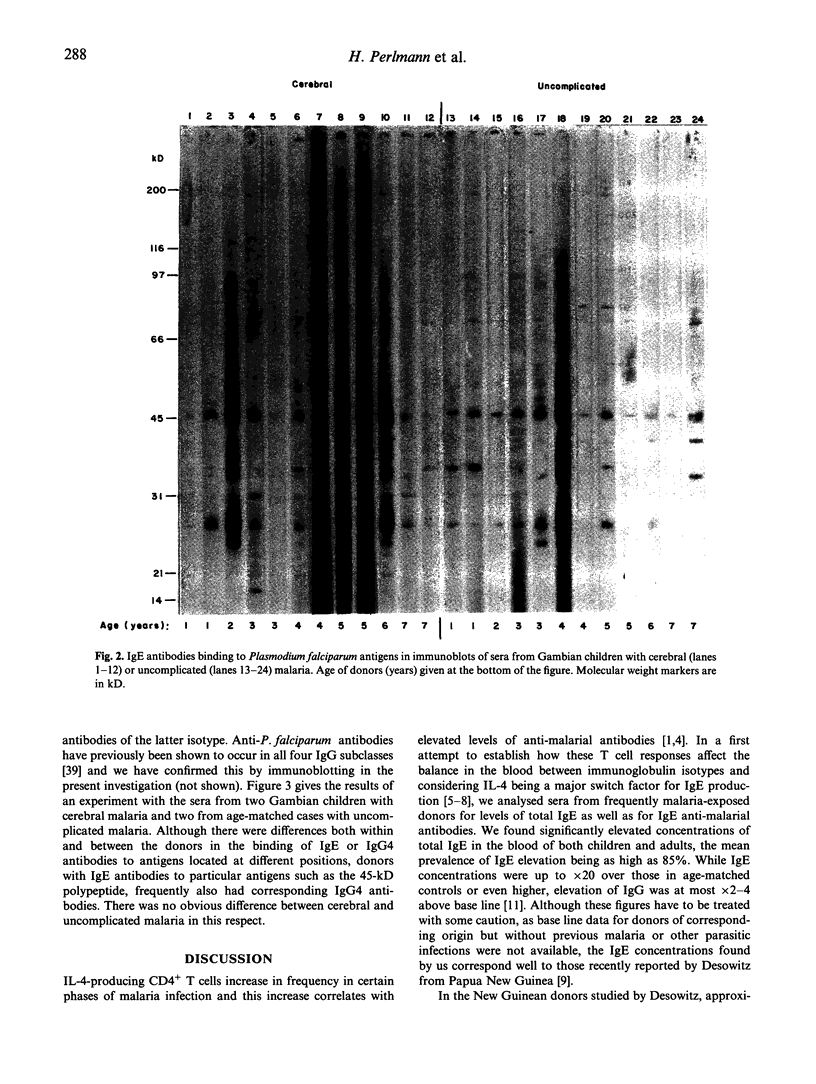
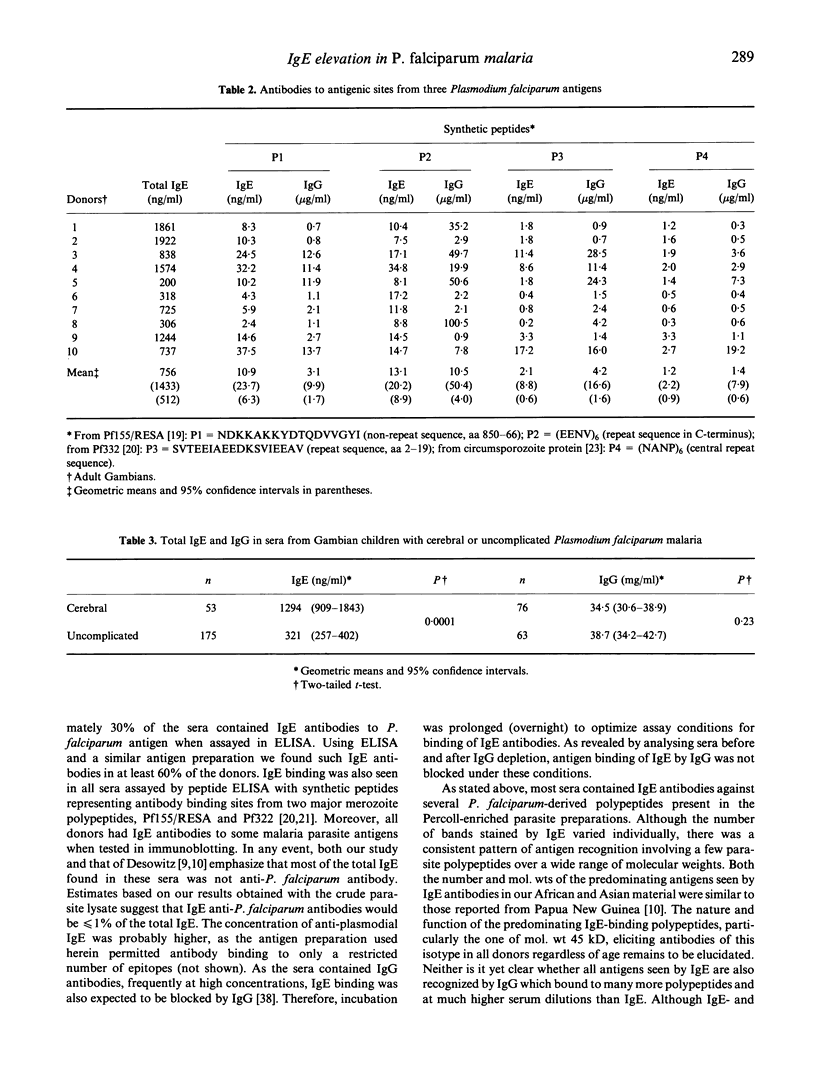
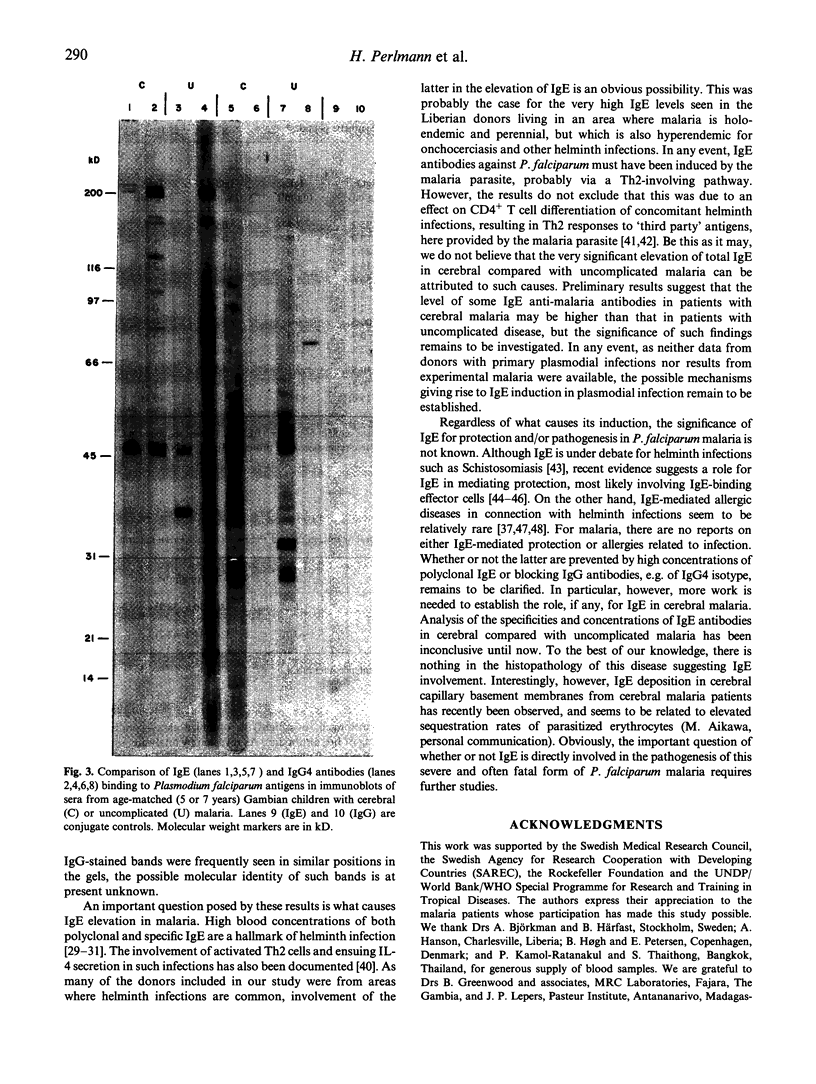
In the course of studying immunoregulation in human Plasmodium falciparum malaria we have investigated IgE levels and IgE anti-plasmodial antibodies in children and adults from areas of high malaria endemicity in both Africa and Asia. On average, 85% of all donors had significantly elevated levels of total IgE. A fraction of the IgE had anti-plasmodial activity as revealed by ELISA with lysates of infected erythrocytes as antigen. Using synthetic peptides representing antigenic regions of two major plasmodial blood stage antigens, IgE antibody concentrations ranged from 5 to 15 ng/ml serum for each of the peptides. On average, the concentrations of the corresponding IgG antibodies were x 500-1000 higher. Immunoblotting of parasite lysates showed that most donors had IgE antibodies against one or several of a restricted number of plasmodial polypeptides, with antibodies against an antigen of mol.wt 45 kD already being present in all donors at an early age. Donors having IgE antibodies to particular antigens also frequently had corresponding IgG4 antibodies, reflecting underlying IL-4-dependent cellular mechanisms controlling formation of these isotypes. As infection with other parasites such as helminths is known to induce IgE elevation, the results do not prove that plasmodial infections were the primary cause of IgE induction. However, the importance of plasmodial infection for IgE elevation was supported by the finding of significantly higher levels of IgE, but not of IgG, in children with cerebral malaria compared with patients with uncomplicated disease.
Full text
PDF


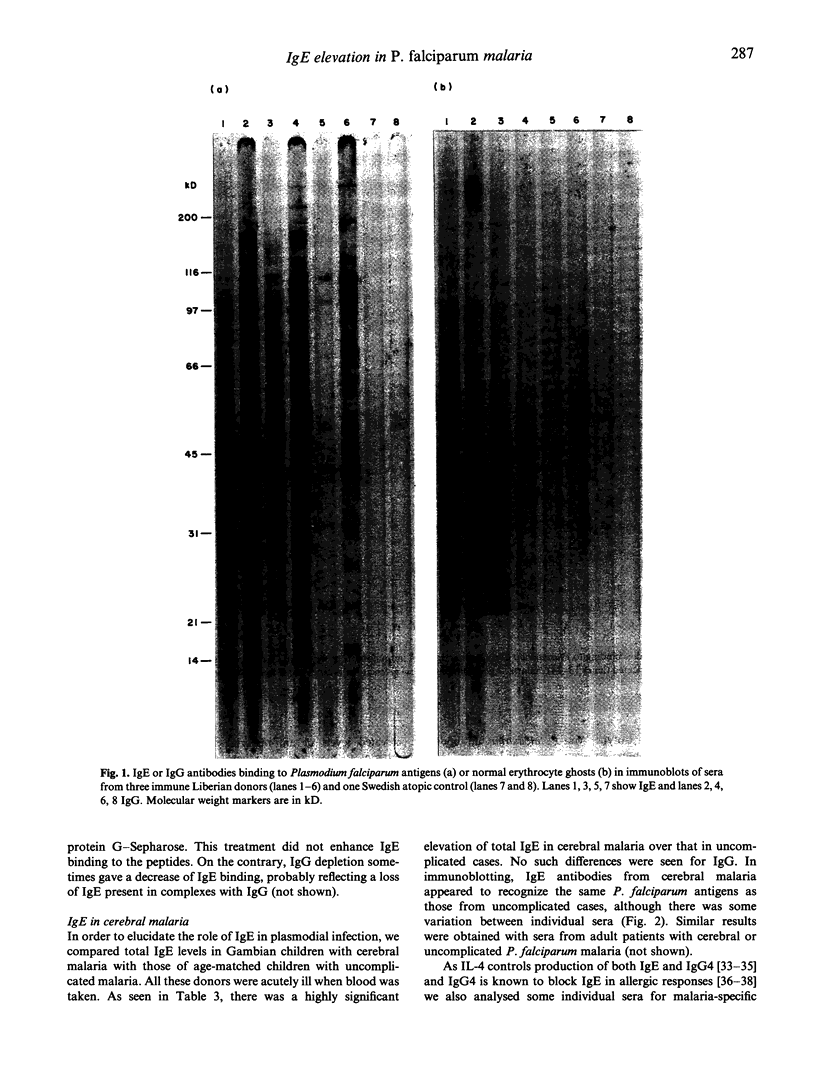


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzins K., Perlmann H., Wåhlin B., Carlsson J., Wahlgren M., Udomsangpetch R., Björkman A., Patarroyo M. E., Perlmann P. Rabbit and human antibodies to a repeated amino acid sequence of a Plasmodium falciparum antigen, Pf 155, react with the native protein and inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1065–1069. doi: 10.1073/pnas.83.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman A., Hedman P., Brohult J., Willcox M., Diamant I., Pehrsson P. O., Rombo L., Bengtsson E. Different malaria control activities in an area of Liberia--effects on malariometric parameters. Ann Trop Med Parasitol. 1985 Jun;79(3):239–246. doi: 10.1080/00034983.1985.11811914. [DOI] [PubMed] [Google Scholar]
- Carlson J., Helmby H., Hill A. V., Brewster D., Greenwood B. M., Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990 Dec 15;336(8729):1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Desowitz R. S., Elm J., Alpers M. P. Plasmodium falciparum-specific immunoglobulin G (IgG), IgM, and IgE antibodies in paired maternal-cord sera from east Sepik Province, Papua New Guinea. Infect Immun. 1993 Mar;61(3):988–993. doi: 10.1128/iai.61.3.988-993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne D. W., Butterworth A. E., Fulford A. J., Kariuki H. C., Langley J. G., Ouma J. H., Capron A., Pierce R. J., Sturrock R. F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992 Jun;22(6):1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Fontenille D., Lepers J. P., Campbell G. H., Coluzzi M., Rakotoarivony I., Coulanges P. Malaria transmission and vector biology in Manarintsoa, high plateaux of Madagascar. Am J Trop Med Hyg. 1990 Aug;43(2):107–115. doi: 10.4269/ajtmh.1990.43.107. [DOI] [PubMed] [Google Scholar]
- Gascan H., Gauchat J. F., Roncarolo M. G., Yssel H., Spits H., de Vries J. E. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991 Mar 1;173(3):747–750. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan P., Blumenthal U. J., Dunn D., Simpson A. J., Wilkins H. A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991 Jan 17;349(6306):243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- Hagel I., Lynch N. R., Pérez M., Di Prisco M. C., López R., Rojas E. Modulation of the allergic reactivity of slum children by helminthic infection. Parasite Immunol. 1993 Jun;15(6):311–315. doi: 10.1111/j.1365-3024.1993.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Hed J., Halldén G., Johansson S. G., Larsson P. Quantitative rather than qualitative differences between monocytes with respect to IgE Fc receptor expression as studied by flow cytofluorometry. Int Arch Allergy Appl Immunol. 1989;88(4):408–411. doi: 10.1159/000234725. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Hussain R., Hamilton R. G., Kumaraswami V., Adkinson N. F., Jr, Ottesen E. A. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981 Oct;127(4):1623–1629. [PubMed] [Google Scholar]
- Hussain R., Ottesen E. A. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J Immunol. 1986 Mar 1;136(5):1859–1863. [PubMed] [Google Scholar]
- Hussain R., Poindexter R. W., Ottesen E. A. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992 May 1;148(9):2731–2737. [PubMed] [Google Scholar]
- Ishizaka A., Sakiyama Y., Nakanishi M., Tomizawa K., Oshika E., Kojima K., Taguchi Y., Kandil E., Matsumoto S. The inductive effect of interleukin-4 on IgG4 and IgE synthesis in human peripheral blood lymphocytes. Clin Exp Immunol. 1990 Mar;79(3):392–396. doi: 10.1111/j.1365-2249.1990.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Kamol-Ratanakul P., Chirakalwasarn N., Lertmaharit S., Dhanamun B., Seublinwong T., Udomsangpetch R., Perlmann H., Perlmann P., Thaithong S. Seroepidemiologic studies of humoral immune response to the Plasmodium falciparum antigens in Thailand. Am J Trop Med Hyg. 1992 Nov;47(5):554–561. doi: 10.4269/ajtmh.1992.47.554. [DOI] [PubMed] [Google Scholar]
- King C. L., Low C. C., Nutman T. B. IgE production in human helminth infection. Reciprocal interrelationship between IL-4 and IFN-gamma. J Immunol. 1993 Mar 1;150(5):1873–1880. [PubMed] [Google Scholar]
- Kullberg M. C., Pearce E. J., Hieny S. E., Sher A., Berzofsky J. A. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992 May 15;148(10):3264–3270. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Gillard S., Simon B., Slade S., Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1(4):416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- Lundgren M., Persson U., Larsson P., Magnusson C., Smith C. I., Hammarström L., Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989 Jul;19(7):1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- Mattei D., Scherf A. The Pf332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene. 1992 Jan 2;110(1):71–79. doi: 10.1016/0378-1119(92)90446-v. [DOI] [PubMed] [Google Scholar]
- Meding S. J., Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol. 1991 Jun;21(6):1433–1438. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- OGILVIE B. M. REAGIN-LIKE ANTIBODIES IN ANIMALS IMMUNE TO HELMINTH PARASITES. Nature. 1964 Oct 3;204:91–92. doi: 10.1038/204091a0. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Berzins K., Wåhlin B., Troye-Blomberg M., Hagstedt M., Andersson I., Högh B., Petersen E., Björkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope specific components. Immunol Rev. 1989 Dec;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Rihet P., Demeure C. E., Bourgois A., Prata A., Dessein A. J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991 Nov;21(11):2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- Rihet P., Demeure C. E., Dessein A. J., Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol. 1992 Aug;22(8):2063–2070. doi: 10.1002/eji.1830220816. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Sjöberg K., Lepers J. P., Raharimalala L., Larsson A., Olerup O., Marbiah N. T., Troye-Blomberg M., Perlmann P. Genetic regulation of human anti-malarial antibodies in twins. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2101–2104. doi: 10.1073/pnas.89.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988 Jan 1;167(1):183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. The role of interleukin-4 in IgE and IgG subclass formation. Springer Semin Immunopathol. 1990;12(4):365–383. doi: 10.1007/BF00225324. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The band 3 protein of the human red cell membrane: a review. J Supramol Struct. 1978;8(3):311–324. doi: 10.1002/jss.400080309. [DOI] [PubMed] [Google Scholar]
- Subba Rao P. V., McCartney-Francis N. L., Metcalfe D. D. An avidin--biotin microELISA for rapid measurement of total and allergen-specific human IgE. J Immunol Methods. 1983 Feb 25;57(1-3):71–85. doi: 10.1016/0022-1759(83)90066-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S. Protective CD4+ T-cell lines raised against Plasmodium chabaudi show characteristics of either Th1 or Th2 cells. Parasite Immunol. 1993 Jun;15(6):301–310. doi: 10.1111/j.1365-3024.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- Taylor T. E., Molyneux M. E., Wirima J. J., Fletcher K. A., Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med. 1988 Oct 20;319(16):1040–1047. doi: 10.1056/NEJM198810203191602. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Olerup O., Larsson A., Sjöberg K., Perlmann H., Riley E., Lepers J. P., Perlmann P. Failure to detect MHC class II associations of the human immune response induced by repeated malaria infections to the Plasmodium falciparum antigen Pf155/RESA. Int Immunol. 1991 Oct;3(10):1043–1051. doi: 10.1093/intimm/3.10.1043. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann H., Patarroyo M. E., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. II. Antigen specific proliferative responses in vitro. Clin Exp Immunol. 1983 Aug;53(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Riley E. M., Kabilan L., Holmberg M., Perlmann H., Andersson U., Heusser C. H., Perlmann P. Production by activated human T cells of interleukin 4 but not interferon-gamma is associated with elevated levels of serum antibodies to activating malaria antigens. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5484–5488. doi: 10.1073/pnas.87.14.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983 Oct;54(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Zwingenberger K., Hohmann A., de Brito M. C., Ritter M. Impaired balance of interleukin-4 and interferon-gamma production in infections with Schistosoma mansoni and intestinal nematodes. Scand J Immunol. 1991 Aug;34(2):243–251. doi: 10.1111/j.1365-3083.1991.tb01543.x. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Gauchat J. F., Aversa G. G., Punnonen J., Gascan H., Yssel H. Regulation of IgE synthesis by cytokines. Curr Opin Immunol. 1991 Dec;3(6):851–858. doi: 10.1016/s0952-7915(05)80003-2. [DOI] [PubMed] [Google Scholar]