Abstract
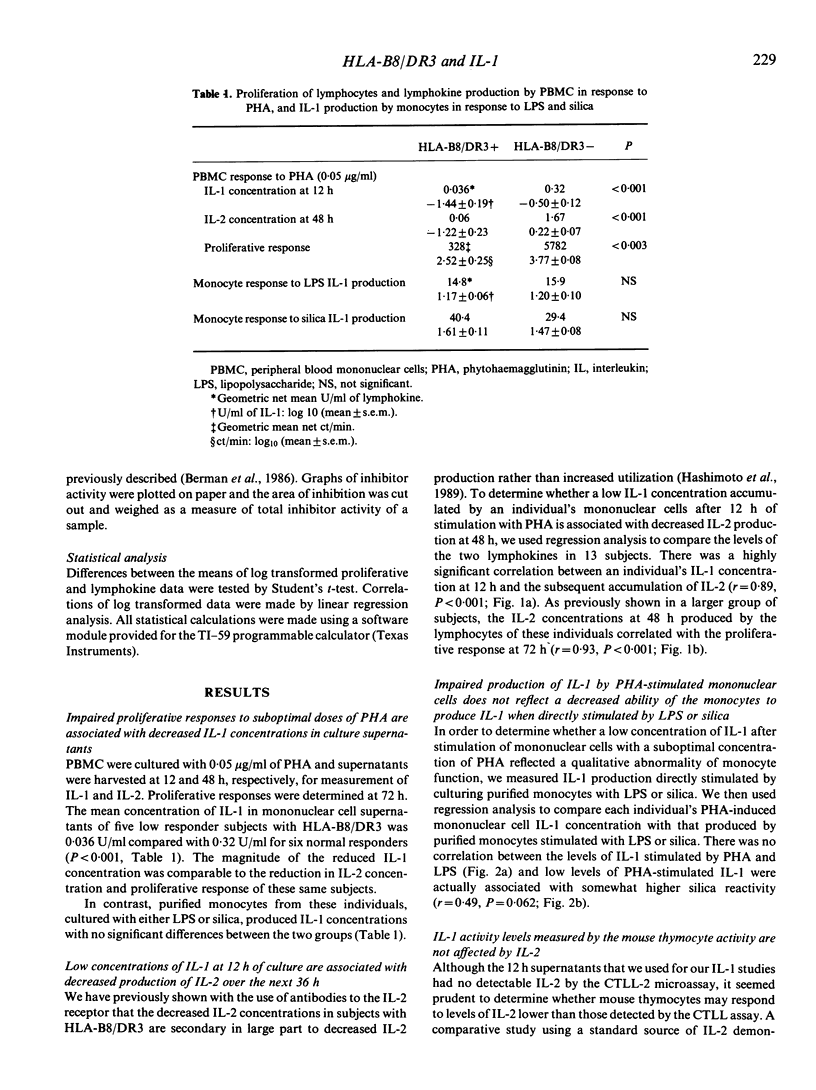
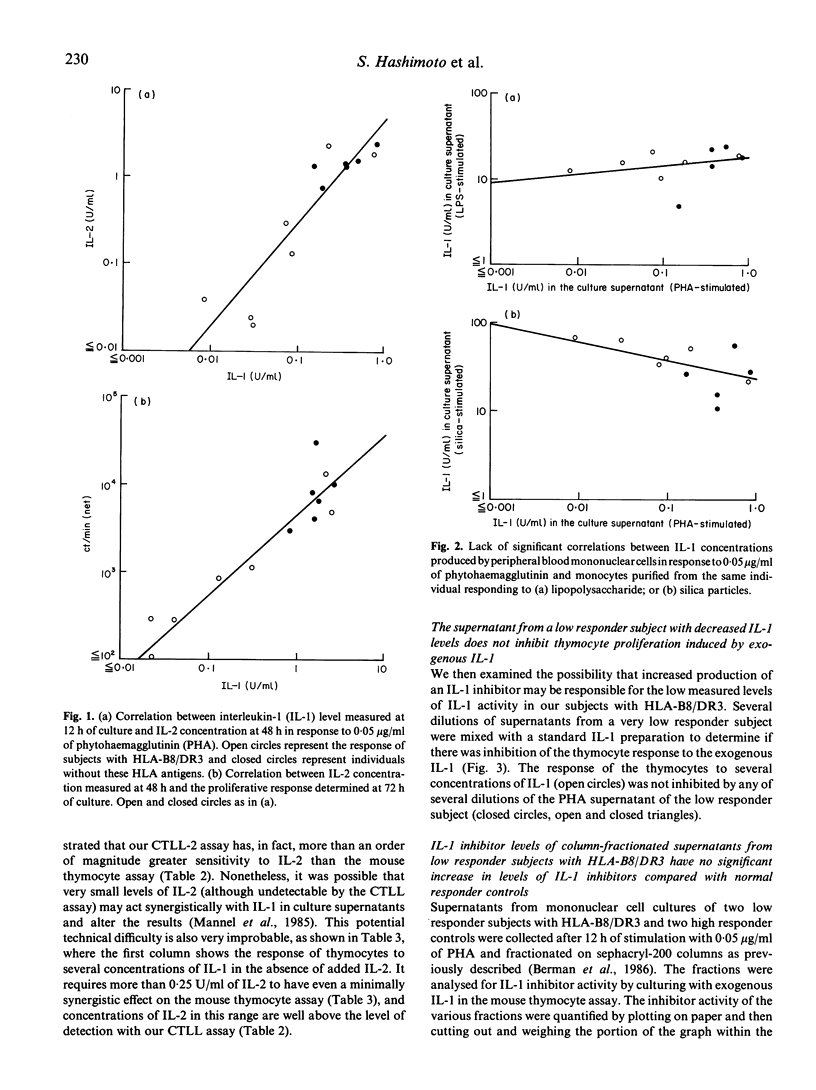
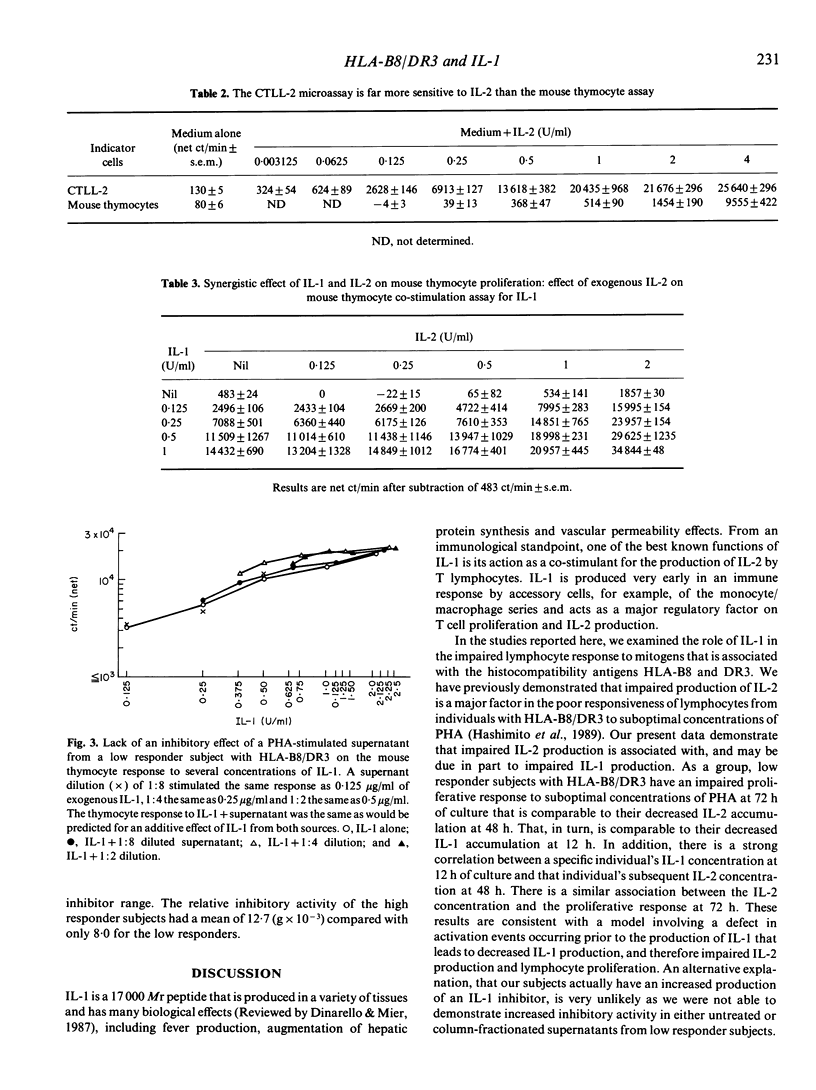
Lymphocytes from normal individuals with the histocompatibility antigens HLA-B8 and DR3 have impaired proliferative responses when stimulated with suboptimal concentrations of mitogens. We have previously shown that an important factor in the impaired response is a failure to produce normal quantities of interleukin-2 (IL-2). To examine the mechanism of decreased responsiveness further, we measured interleukin-1 (IL-1) production of low responder subjects compared with controls. The peripheral blood mononuclear cells of five low responder individuals with HLA-B8/DR3 stimulated with 0.05 micrograms/ml of phytohaemagglutinin (PHA) accumulated only 0.036 U/ml of IL-1 compared with 0.32 U/ml for normal responders. There was a highly significant correlation between the PHA-stimulated IL-1 concentration at 12 h and the subsequent IL-2 concentration at 48 h(r = 0.89, P less than 0.0001) suggesting a role of decreased IL-1 production in the impaired response. A study of unfractionated or column-fractionated culture supernatants revealed no evidence that the decreased IL-1 activity in the supernatants of low responder subjects was related to increased IL-1 inhibitor concentrations. These results suggest that impaired IL-2 production and lymphocyte proliferation in healthy subjects with HLA-B8/DR3 may be mediated at least in part by decreased IL-1 production, and implicates a defect of a very early event in lymphocyte activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambinder J. N., Chiorazzi N., Gibofsky A., Fotino M., Kunkel H. G. Special characteristics of cellular immune function in normal individuals of the HLA-DR3 type. Clin Immunol Immunopathol. 1982 May;23(2):269–274. doi: 10.1016/0090-1229(82)90113-1. [DOI] [PubMed] [Google Scholar]
- Amer A., Singh G., Darke C., Dolby A. E. Impaired lymphocyte responsiveness to phytohaemagglutinin associated with the possession of HLA-B8/DR3. Tissue Antigens. 1986 Oct;28(4):193–198. doi: 10.1111/j.1399-0039.1986.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F., Virelizier J. L., Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985 Apr;134(4):2444–2448. [PubMed] [Google Scholar]
- Berman M. A., Sandborg C. I., Calabia B. S., Andrews B. S., Friou G. J. Studies of an interleukin 1 inhibitor: characterization and clinical significance. Clin Exp Immunol. 1986 Apr;64(1):136–145. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hashimoto S., McCombs C. C., Michalski J. P. Mechanism of a lymphocyte abnormality associated with HLA-B8/DR3 in clinically healthy individuals. Clin Exp Immunol. 1989 Jun;76(3):317–323. [PMC free article] [PubMed] [Google Scholar]
- Kallenberg C. G., Van der Voort-Beelen J. M., D'Amaro J., The T. H. Increased frequency of B8/DR3 in scleroderma and association of the haplotype with impaired cellular immune response. Clin Exp Immunol. 1981 Mar;43(3):478–485. [PMC free article] [PubMed] [Google Scholar]
- Lawley T. J., Hall R. P., Fauci A. S., Katz S. I., Hamburger M. I., Frank M. M. Defective Fc-receptor functions associated with the HLA-B8/DRw3 haplotype: studies in patients with dermatitis herpetiformis and normal subjects. N Engl J Med. 1981 Jan 22;304(4):185–192. doi: 10.1056/NEJM198101223040401. [DOI] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- McCombs C. C., Michalski J. P. Lymphocyte abnormality associated with HLA-B8 in healthy young adults. J Exp Med. 1982 Sep 1;156(3):936–941. doi: 10.1084/jem.156.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombs C. C., Michalski J. P., deShazo R., Bozelka B., Lane J. T. Immune abnormalities associated with HLA-B8: lymphocyte subsets and functional correlates. Clin Immunol Immunopathol. 1986 Apr;39(1):112–120. doi: 10.1016/0090-1229(86)90210-2. [DOI] [PubMed] [Google Scholar]
- McCombs C., Michalski J. P., Talal N. Cellular interactions in lymphocyte proliferation: effect of syngeneic and xenogeneic macrophages. Cell Immunol. 1976 May;23(2):283–296. doi: 10.1016/0008-8749(76)90194-5. [DOI] [PubMed] [Google Scholar]
- McKenna R., Stevens F. M., McNicholl B., Scholz S., Albert E., McCarthy C. F. Family and population studies of HLA and coeliac disease in the West of Ireland. Tissue Antigens. 1983 Sep;22(3):175–181. doi: 10.1111/j.1399-0039.1983.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. Studies on the purification and structure-functional relationships of murine lymphocyte activating factor (Interleukin 1). Mol Immunol. 1980 May;17(5):571–577. doi: 10.1016/0161-5890(80)90155-8. [DOI] [PubMed] [Google Scholar]
- Repo H., Jättelä M., Leirisalo-Repo M., Hurme M. Production of tumour necrosis factor and interleukin 1 by monocytes of patients with previous Yersinia arthritis. Clin Exp Immunol. 1988 Jun;72(3):410–414. [PMC free article] [PubMed] [Google Scholar]
- Rood J. J., Hooff J. P., Keuning J. J. Disease predisposition, immune responsiveness and the fine structure of the HL-A supergene. A need for a reappraisal. Transplant Rev. 1975;22:75–104. doi: 10.1111/j.1600-065x.1975.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Roosnek E. E., Brouwer M. C., Aarden L. A. T cell triggering by lectins. I. Requirements for interleukin 2 production; lectin concentration determines the accessory cell dependency. Eur J Immunol. 1985 Jul;15(7):652–656. doi: 10.1002/eji.1830150703. [DOI] [PubMed] [Google Scholar]
- Sandborg C. I., Berman M. A., Andrews B. S., Friou G. J. Interleukin-1 production by mononuclear cells from patients with scleroderma. Clin Exp Immunol. 1985 May;60(2):294–302. [PMC free article] [PubMed] [Google Scholar]
- Terasaki P. I., Bernoco D., Park M. S., Ozturk G., Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol. 1978 Feb;69(2):103–120. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. T cell induction of membrane IL 1 on macrophages. J Immunol. 1986 Dec 15;137(12):3868–3873. [PubMed] [Google Scholar]


