Abstract
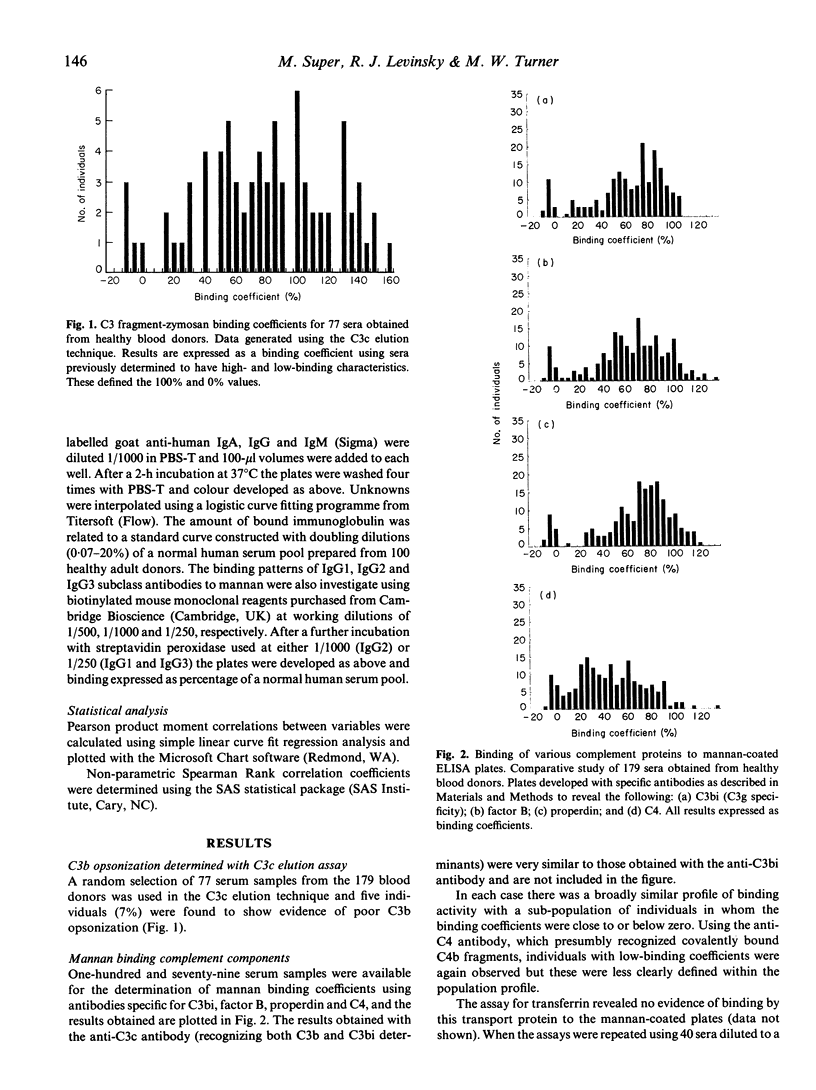
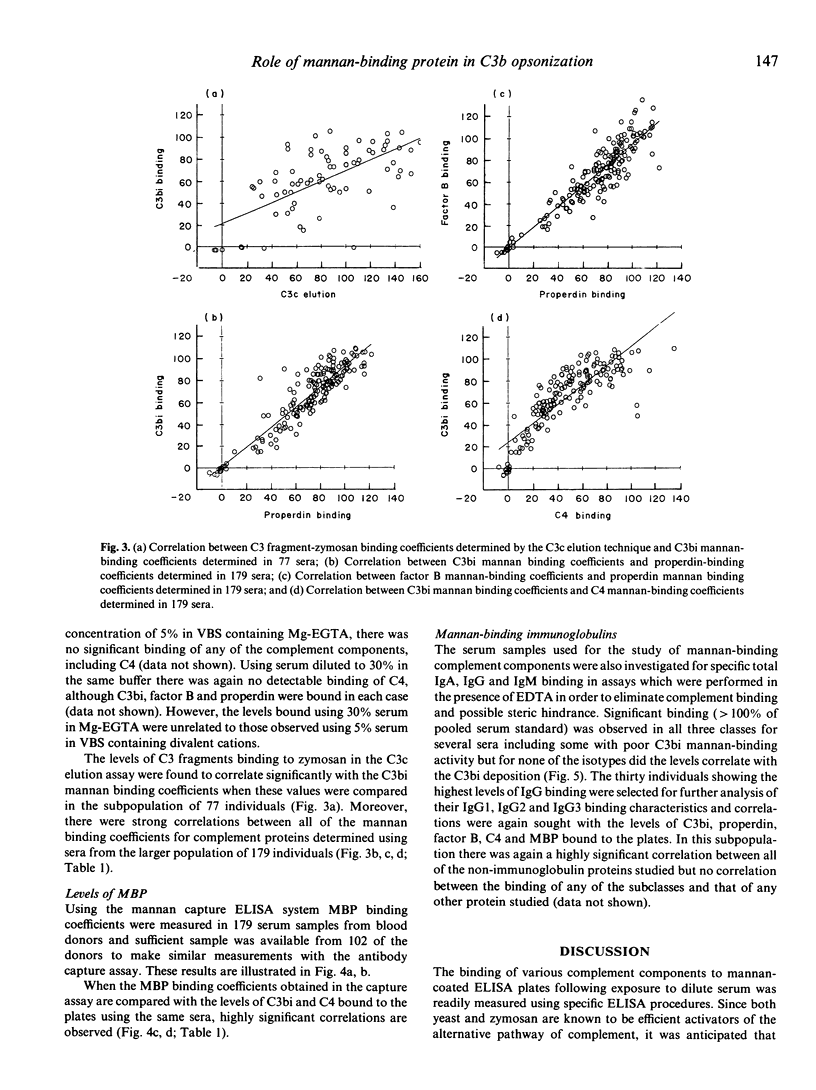
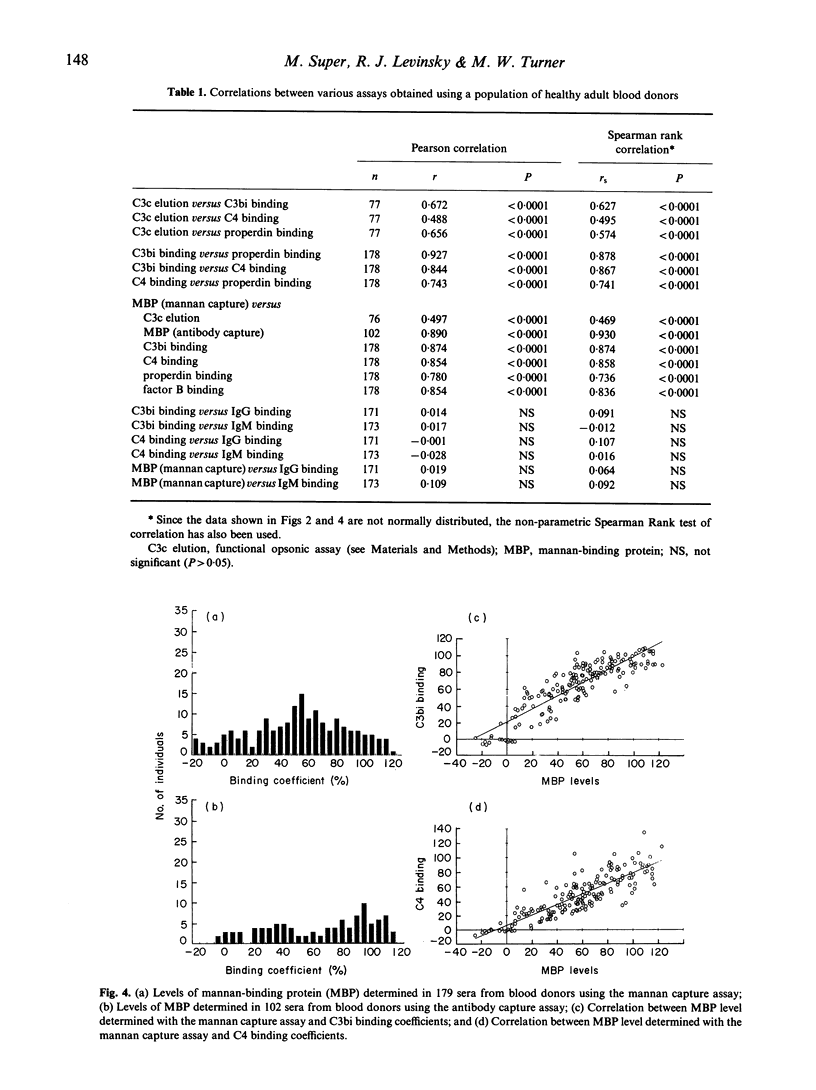
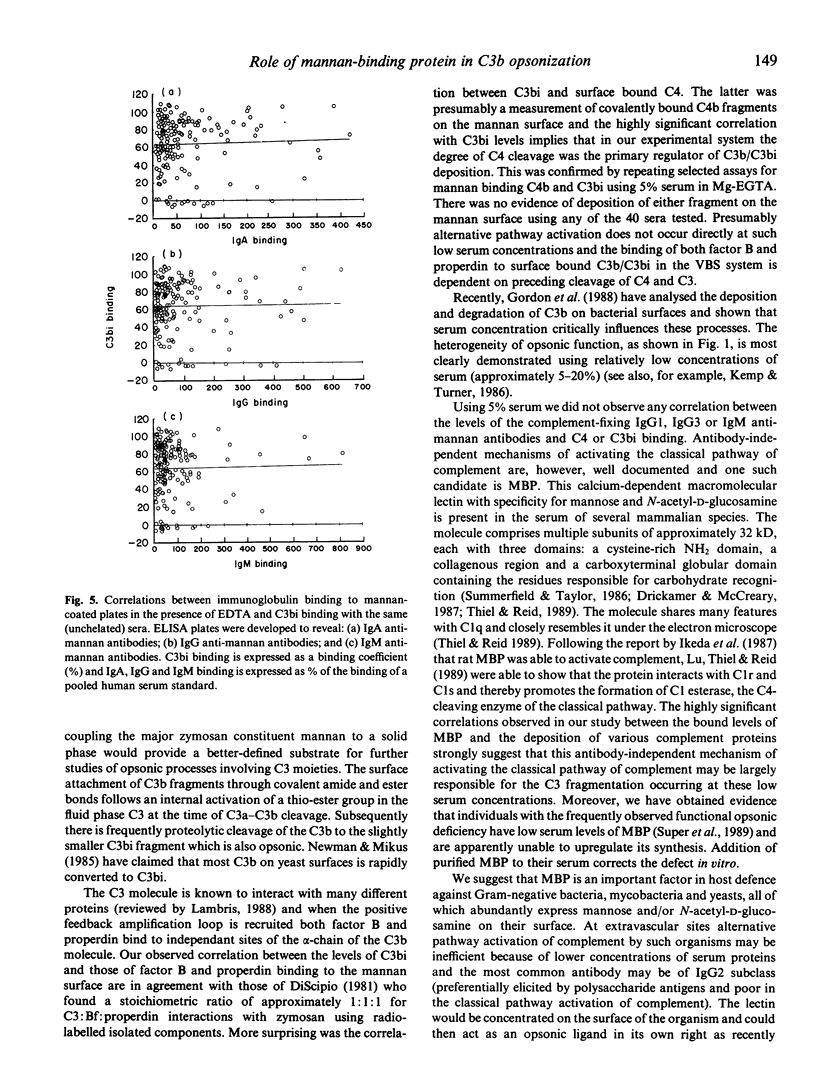
When sera diluted to 5% in a buffer containing calcium and magnesium were incubated with mannan-coated ELISA plates, C4 fragments, properdin and factor B were bound to the plates as well as the expected opsonic C3 fragments, C3b and C3bi. The calcium-dependent lectin mannan-binding protein, which is structurally similar to C1q, was also shown to bind in this assay and analysis of sera from 179 healthy blood donors revealed that the binding levels of all these proteins were highly significantly correlated. Results obtained with a previously described C3b opsonic assay using zymosan also correlated with the mannan-binding levels. When the sera were diluted to 5% in the presence of Mg-EGTA there was no detectable binding of complement proteins to the mannan surface, confirming that no alternative pathway activation occurred at this serum concentration. When sera were diluted to 5% in a buffer containing EDTA in order to study immunoglobulin binding in the absence of complement activation, the levels of bound IgG1, IgG2, IgG3, IgA and IgM antibodies were found to be completely unrelated to the C3bi binding levels previously observed. The results suggest that in this experimental system using low concentrations of serum, mannan-binding protein initiates an antibody-independent mechanism of cleavage of the classical pathway component C4, which subsequently regulates the degree of cleavage of C3 and recruitment of alternative pathway proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. S., Farmer V. C., Jones D., Taylor I. F. The glucan components of the cell wall of baker's yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem J. 1969 Sep;114(3):557–567. doi: 10.1042/bj1140557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiScipio R. G. The binding of human complement proteins C5, factor B, beta 1H and properdin to complement fragment C3b on zymosan. Biochem J. 1981 Dec 1;199(3):485–496. doi: 10.1042/bj1990485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K., McCreary V. Exon structure of a mannose-binding protein gene reflects its evolutionary relationship to the asialoglycoprotein receptor and nonfibrillar collagens. J Biol Chem. 1987 Feb 25;262(6):2582–2589. [PubMed] [Google Scholar]
- Gordon D. L., Rice J., Finlay-Jones J. J., McDonald P. J., Hostetter M. K. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J Infect Dis. 1988 Apr;157(4):697–704. doi: 10.1093/infdis/157.4.697. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Sannoh T., Kawasaki N., Kawasaki T., Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987 Jun 5;262(16):7451–7454. [PubMed] [Google Scholar]
- Kemp A. S., Turner M. W. The role of opsonins in vacuolar sealing and the ingestion of zymosan by human neutrophils. Immunology. 1986 Sep;59(1):69–74. [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Falconer J. S., Bashey A., Beck J. S. The effect of C3 levels on yeast opsonization by normal and pathological sera: identification of a complement independent opsonin. Clin Exp Immunol. 1983 Dec;54(3):793–800. [PMC free article] [PubMed] [Google Scholar]
- Kuhlman M., Joiner K., Ezekowitz R. A. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989 May 1;169(5):1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D. The multifunctional role of C3, the third component of complement. Immunol Today. 1988 Dec;9(12):387–393. doi: 10.1016/0167-5699(88)91240-6. [DOI] [PubMed] [Google Scholar]
- Levinsky R. J., Harvey B. A., Paleja S. A rapid objective method for measuring the yeast opsonisation activity of serum. J Immunol Methods. 1978;24(3-4):251–256. doi: 10.1016/0022-1759(78)90129-1. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Mikus L. K. Deposition of C3b and iC3b onto particulate activators of the human complement system. Quantitation with monoclonal antibodies to human C3. J Exp Med. 1985 Jun 1;161(6):1414–1431. doi: 10.1084/jem.161.6.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- Richardson V. F., Larcher V. F., Price J. F. A common congenital immunodeficiency predisposing to infection and atopy in infancy. Arch Dis Child. 1983 Oct;58(10):799–802. doi: 10.1136/adc.58.10.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson V. F., Larcher V. F., Price J. F. Yeast opsonization in newborn infants and its relationship to parental atopy. Clin Exp Immunol. 1982 May;48(2):411–416. [PMC free article] [PubMed] [Google Scholar]
- Roberton D. M., Dhanjal N. K., Levinsky R. J., Mowbray J. F., Turner M. W. Polymorphonuclear neutrophil iodination response as an estimate of defective yeast opsonization. Clin Exp Immunol. 1981 Jan;43(1):208–214. [PMC free article] [PubMed] [Google Scholar]
- Soothill J. F., Harvey B. A. Defective opsonization. A common immunity deficiency. Arch Dis Child. 1976 Feb;51(2):91–99. doi: 10.1136/adc.51.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield J. A., Taylor M. E. Mannose-binding proteins in human serum: identification of mannose-specific immunoglobulins and a calcium-dependent lectin, of broader carbohydrate specificity, secreted by hepatocytes. Biochim Biophys Acta. 1986 Sep 4;883(2):197–206. doi: 10.1016/0304-4165(86)90309-0. [DOI] [PubMed] [Google Scholar]
- Super M., Thiel S., Lu J., Levinsky R. J., Turner M. W. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989 Nov 25;2(8674):1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- Thiel S., Reid K. B. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989 Jun 19;250(1):78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- Turner M. W., Mowbray J. F., Roberton D. R. A study of C3b deposition on yeast surfaces by sera of known opsonic potential. Clin Exp Immunol. 1981 Nov;46(2):412–419. [PMC free article] [PubMed] [Google Scholar]
- Turner M. W., Seymour N. D., Kazatchkine M. D., Mowbray J. F. Suboptimal C3b/C3bi deposition and defective yeast opsonization. I. Evidence for the absence of essential co-factor activity. Clin Exp Immunol. 1985 Nov;62(2):427–434. [PMC free article] [PubMed] [Google Scholar]
- Yeaman G. R., Kerr M. A. Opsonization of yeast by human serum IgA anti-mannan antibodies and phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1987 Apr;68(1):200–208. [PMC free article] [PubMed] [Google Scholar]


