Abstract
Patients with systemic lupus erythematosus (SLE) experience clinical flares in association with superimposed bacterial infection. To investigate whether heightened immune phenomena during the course of bacterial infections were related to abnormal disposal of immune complexes, we administered bacterial lipopolysaccharide (LPS) to C57BL/6 mice for 5 weeks. Control mice received vehicle only. We then challenged the mice with a subsaturating dose of radiolabelled immune complexes intravenously and determined the localization of immune complexes in liver, spleen and kidney. In comparison to control mice, mice exposed to LPS developed features of polyclonal B cell activation, autoimmune phenomena, delayed removal of immune complexes from the circulation, diminished liver uptake of immune complexes, and enhanced localization of immune complexes in the kidneys. The findings could not be attributed to biological processes dependent on complement concentration. Instead, interferences with Fc receptor function, or with endocytosis of immune complexes may represent likely possibilities. Thus, clinical flares in patients with SLE, in the presence of a superimposed infection, may result from enhanced localization of immune complexes in organs due to altered mechanisms of their disposal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Kramer S. B., Radin A., Holzman R. Salmonella bacteremia in systemic lupus erythematosus. Eight-year experience at a municipal hospital. Arthritis Rheum. 1985 Jan;28(1):75–79. doi: 10.1002/art.1780280112. [DOI] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. The switch from IgM to IgG secretion in single mitogen-stimulated B-cell clones. J Exp Med. 1978 Jun 1;147(6):1744–1754. doi: 10.1084/jem.147.6.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockow B., Mannik M. Clearance and tissue uptake of immune complexes in complement-depleted and control mice. Immunology. 1981 Apr;42(4):497–504. [PMC free article] [PubMed] [Google Scholar]
- Cavallo T., Goldman M., Graves K., Lambert P. H. Altered glomerular permeability in the early phase of immune complex nephritis. Kidney Int. 1983 Nov;24(5):632–637. doi: 10.1038/ki.1983.204. [DOI] [PubMed] [Google Scholar]
- Cavallo T., Goldman M., Lambert P. H. Animal model of human disease. Proliferative glomerulonephritis associated with polyclonal B-cell activation. Am J Pathol. 1984 Feb;114(2):346–348. [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- De Waal R. M., Schrijver G., Bogman M. J., Assmann K. J., Koene R. A. An improved sensitive and simple microassay of mouse complement. J Immunol Methods. 1988 Apr 6;108(1-2):213–221. doi: 10.1016/0022-1759(88)90422-x. [DOI] [PubMed] [Google Scholar]
- Finbloom D. S. Binding, endocytosis, and degradation of model immune complexes by murine macrophages at various levels of activation. Clin Immunol Immunopathol. 1985 Sep;36(3):275–288. doi: 10.1016/0090-1229(85)90048-0. [DOI] [PubMed] [Google Scholar]
- Finbloom D. S., Plotz P. H. Studies of reticuloendothelial function in the mouse with model immune complexes. I. Serum clearance and tissue uptake in normal C3H mice. J Immunol. 1979 Oct;123(4):1594–1599. [PubMed] [Google Scholar]
- Fournié G. J., Mignon-Conté M. A., Lulé J., Gayral-Ta Minh M., Haas S., Bauriaud R., Conté J. J. Immune complex glomerulonephritis in mice infected with Escherichia coli. Clin Exp Immunol. 1980 Oct;42(1):77–85. [PMC free article] [PubMed] [Google Scholar]
- Haakenstad A. O., Case J. B., Mannik M. Effect of cortisone on the disappearance kinetics and tissue localization of soluble immune complexes. J Immunol. 1975 Apr;114(4):1153–1160. [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974 May;112(5):1939–1948. [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. The disappearance kinetics of soluble immune complexes prepared with reduced and alkylated antibodies and with intact antibodies in mice. Lab Invest. 1976 Sep;35(3):283–292. [PubMed] [Google Scholar]
- Haakenstad A. O., Striker G. E., Mannik M. The disappearance kinetics and glomerular deposition of small-latticed soluble immune complexes. Immunology. 1982 Nov;47(3):407–414. [PMC free article] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. Subclass-restricted IgG polyclonal antibody production in mice injected with lipid A-rich lipopolysaccharides. J Exp Med. 1981 Feb 1;153(2):324–338. doi: 10.1084/jem.153.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Fournié G. J., Türler H., Miescher P. A. Features of systemic lupus erythematosus in mice injected with bacterial lipopolysaccharides: identificantion of circulating DNA and renal localization of DNA-anti-DNA complexes. J Exp Med. 1977 May 1;145(5):1115–1130. doi: 10.1084/jem.145.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. Determination of anti-DNA antibodies by a modified 125I-labelled DNA-binding test. Elimination of non-specific binding of DNA to non-immunoglobulin basic proteins by using an anionic detergent. Clin Exp Immunol. 1976 Dec;26(3):425–430. [PMC free article] [PubMed] [Google Scholar]
- Jimenez R. A., Haakenstad A. O., Mannik M. Hepatic uptake of small-latticed immune complexes does not alter mononuclear phagocyte system function. Immunology. 1983 Feb;48(2):205–210. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magilavy D. B., Rifai A., Plotz P. H. An abnormality of immune complex kinetics in murine lupus. J Immunol. 1981 Feb;126(2):770–774. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
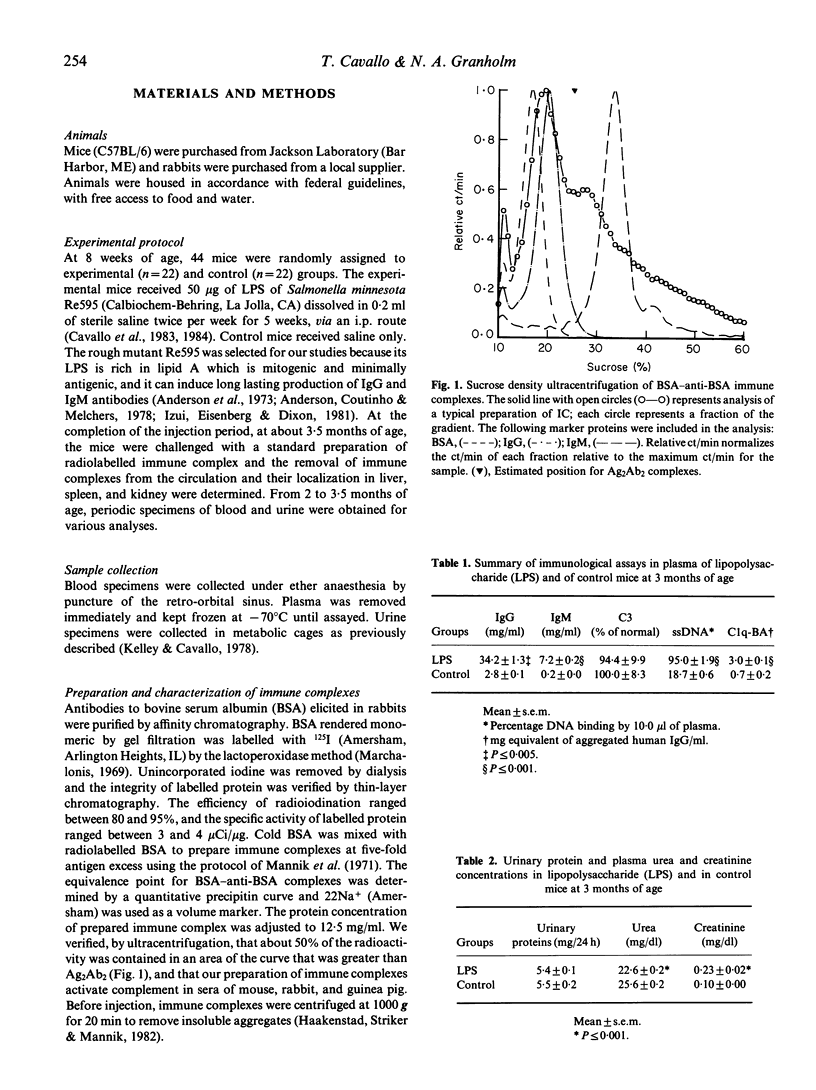
- Mannik M., Arend M. P., Hall A. P., Gilliland B. C. Studies on antigen-antibody complexes. I. Elimination of soluble complexes from rabbit circulation. J Exp Med. 1971 Apr 1;133(4):713–739. doi: 10.1084/jem.133.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Steinberg A. D., Green I., Nussenzweig V. Complement-dependent alterations in the handling of immune complexes by NZB/W mice. J Immunol. 1975 Apr;114(4):1166–1170. [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Pesce A. J. Methods used for the analysis of proteins in the urine. Nephron. 1974;13(1):93–104. doi: 10.1159/000180371. [DOI] [PubMed] [Google Scholar]
- Rifai A., Mannik M. Clearance kinetics and fate of mouse IgA immune complexes prepared with monomeric or dimeric IgA. J Immunol. 1983 Apr;130(4):1826–1832. [PubMed] [Google Scholar]
- Severinson E., Bergstedt-Lindqvist S., van der Loo W., Fernandez C. Characterization of the IgG response induced by polyclonal B cell activators. Immunol Rev. 1982;67:73–85. doi: 10.1111/j.1600-065x.1982.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Sakane T. Induction of excessive B cell proliferation and differentiation by an in vitro stimulus in culture in human systemic lupus erythematosus. J Clin Invest. 1989 Mar;83(3):937–944. doi: 10.1172/JCI113979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]


