Abstract
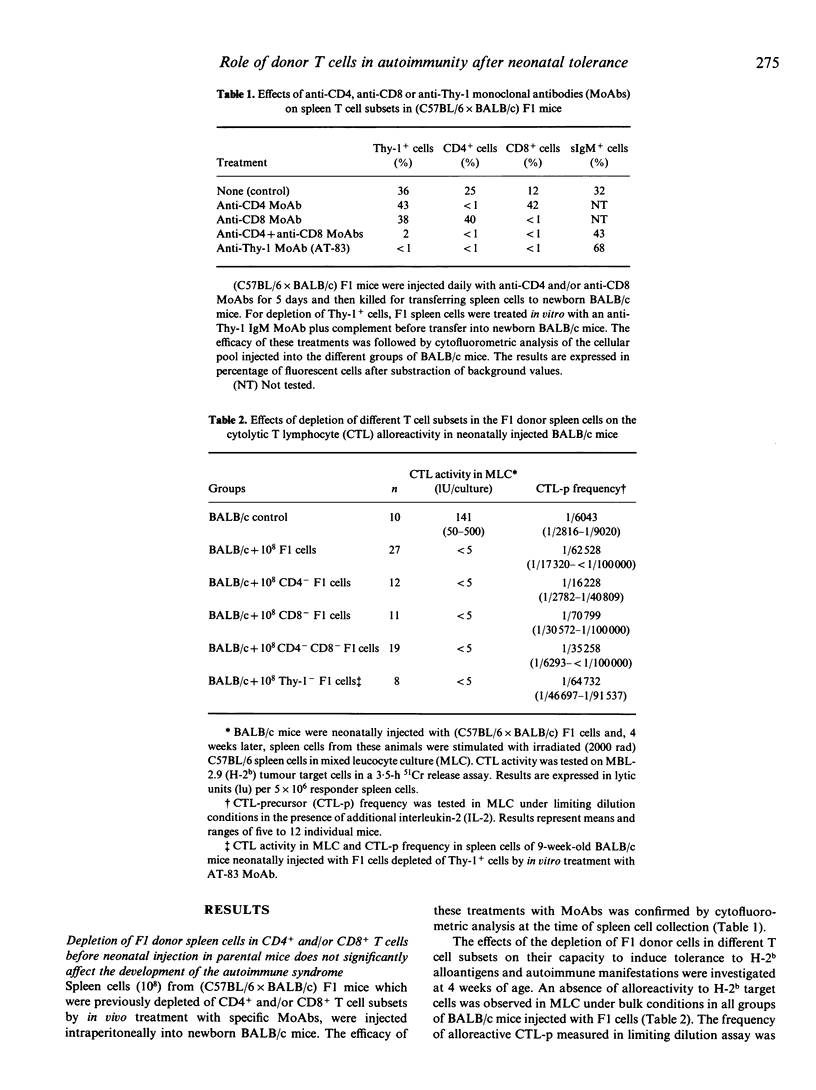
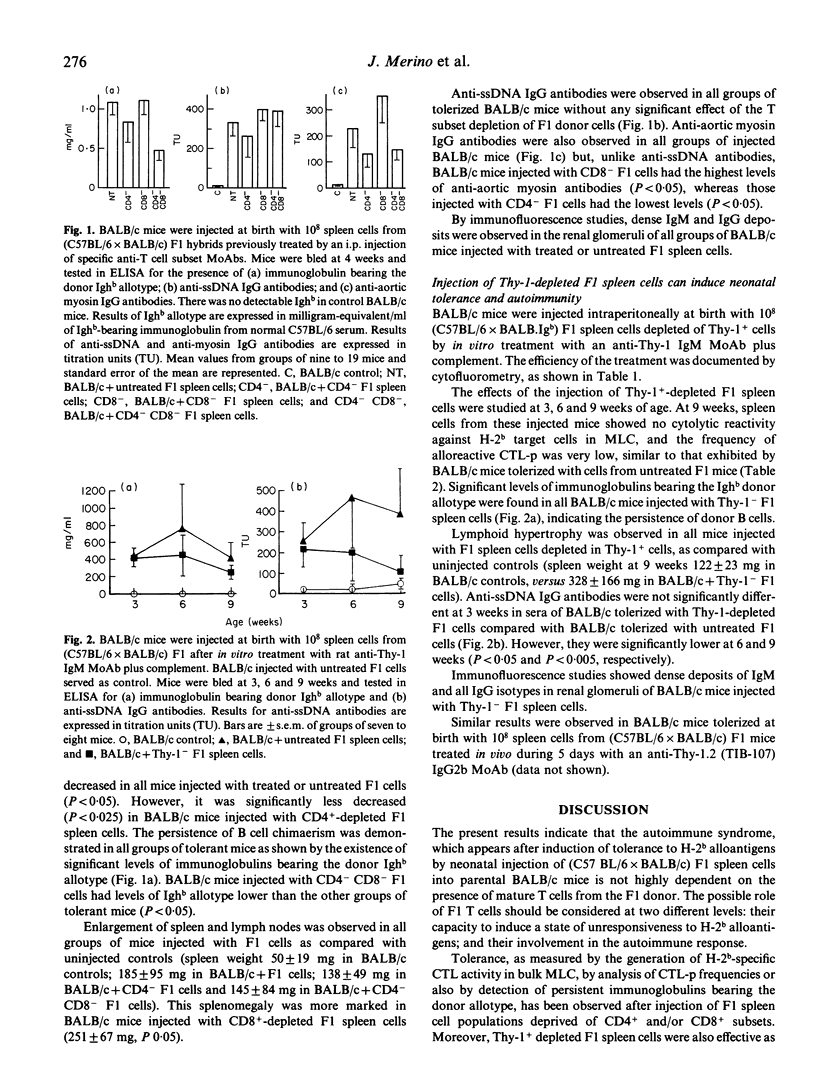
The injection of (C57BL/6 x BALB/c) F1 spleen cells into BALB/c newborn mice leads to activation of persisting F1 donor B cells and development of a lupus-like syndrome in tolerized BALB/c mice. This syndrome is characterized by hypergammaglobulinaemia, high levels of anti-DNA and anti-Sm antibodies, circulating immune complexes and deposits of immunoglobulin in renal glomeruli. The role of donor T cells in this model was investigated by injecting the newborn mice with F1 cells depleted in different T cell subsets by using specific monoclonal antibodies (MoAbs). Tolerance, as shown by an absence of H-2b-specific CTL alloreactivity and persistence of immunoglobulin bearing the donor allotype were observed in mice injected with F1 cells previously depleted in the CD4+ and/or CD8+ T cell subsets as well as in those which received Thy-1+-depleted F1 spleen cells. In these mice, a typical autoimmune syndrome was found, including splenomegaly and lymphadenopathy, anti-ssDNA and anti-aortic myosin IgG antibodies and renal deposition of immunoglobulin. However, some quantitative changes were seen: the levels of anti-aortic myosin antibodies were lower in mice tolerized with CD4+-depleted F1 cells than in those receiving untreated F1 cells. Conversely, higher levels of these autoantibodies were observed in mice tolerized with CD8+-depleted F1 cells. These results suggest that mature donor T cells are not necessary neither for the establishment of neonatal tolerance to alloantigens nor for the activation of F1 donor B cells in the production of the autoimmune syndrome in tolerant mice, but they may contribute in the regulation of the expression of autoreactive B cell clones.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Feng H. M., Glasebrook A. L., Engers H. D., Louis J. A. Clonal analysis of T cell unresponsiveness to alloantigens induced by neonatal injection of F1 spleen cells into parental mice. J Immunol. 1983 Nov;131(5):2165–2169. [PubMed] [Google Scholar]
- Goldman M., Feng H. M., Engers H., Hochman A., Louis J., Lambert P. H. Autoimmunity and immune complex disease after neonatal induction of transplantation tolerance in mice. J Immunol. 1983 Jul;131(1):251–258. [PubMed] [Google Scholar]
- Hard R. C., Jr, Kullgren B. Etiology, pathogenesis, and prevention of a fatal host-versus-graft syndrome in parent-F1 mouse chimeras. Am J Pathol. 1970 May;59(2):203–224. [PMC free article] [PubMed] [Google Scholar]
- Ishizaka S. T., Carnaud C., Stutman O. Differential susceptibility of cytotoxic and helper T cell precursors to neonatal tolerization to histocompatibility antigens. J Immunol. 1986 Oct 1;137(7):2093–2099. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Luzuy S., Merino J., Engers H., Izui S., Lambert P. H. Autoimmunity after induction of neonatal tolerance to alloantigens: role of B cell chimerism and F1 donor B cell activation. J Immunol. 1986 Jun 15;136(12):4420–4426. [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Merino J., Schurmans S., Duchosal M. A., Izui S., Lambert P. H. Autoimmune syndrome after induction of neonatal tolerance to alloantigens. CD4+ T cells from the tolerant host activate autoreactive F1 B cells. J Immunol. 1989 Oct 1;143(7):2202–2208. [PubMed] [Google Scholar]
- Merino J., Schurmans S., Luzuy S., Izui S., Vassalli P., Lambert P. H. Autoimmune syndrome after induction of neonatal tolerance to alloantigens: effects of in vivo treatment with anti-T cell subset monoclonal antibodies. J Immunol. 1987 Sep 1;139(5):1426–1431. [PubMed] [Google Scholar]
- Miller R. G. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980 Oct 9;287(5782):544–546. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- Muraoka S., Miller R. G. Cells in bone marrow and in T cell colonies grown from bone marrow can suppress generation of cytotoxic T lymphocytes directed against their self antigens. J Exp Med. 1980 Jul 1;152(1):54–71. doi: 10.1084/jem.152.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Thomas S. M., Niederman R. Human platelet myosin. I. Purification by a rapid method applicable to other nonmuscle cells. Anal Biochem. 1974 Jul;60(1):258–266. doi: 10.1016/0003-2697(74)90152-3. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R. P., Bevan M. J. Split tolerance induced by the intrathymic adoptive transfer of thymocyte stem cells. J Exp Med. 1988 Jul 1;168(1):143–156. doi: 10.1084/jem.168.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M., Kondo N., Itoh T., Yoshiki T. Autoimmune disease and malignant lymphoma associated with host-versus-graft disease in mice. Clin Exp Immunol. 1985 Dec;62(3):535–544. [PMC free article] [PubMed] [Google Scholar]
- Weissman I. L., Jerabek L., Greenspan S. Tolerance and the H-Y antigen: Requirement for male T cells, but not B cells, to induce tolerance in neonatal female mice. Transplantation. 1984 Jan;37(1):3–6. [PubMed] [Google Scholar]


