Abstract
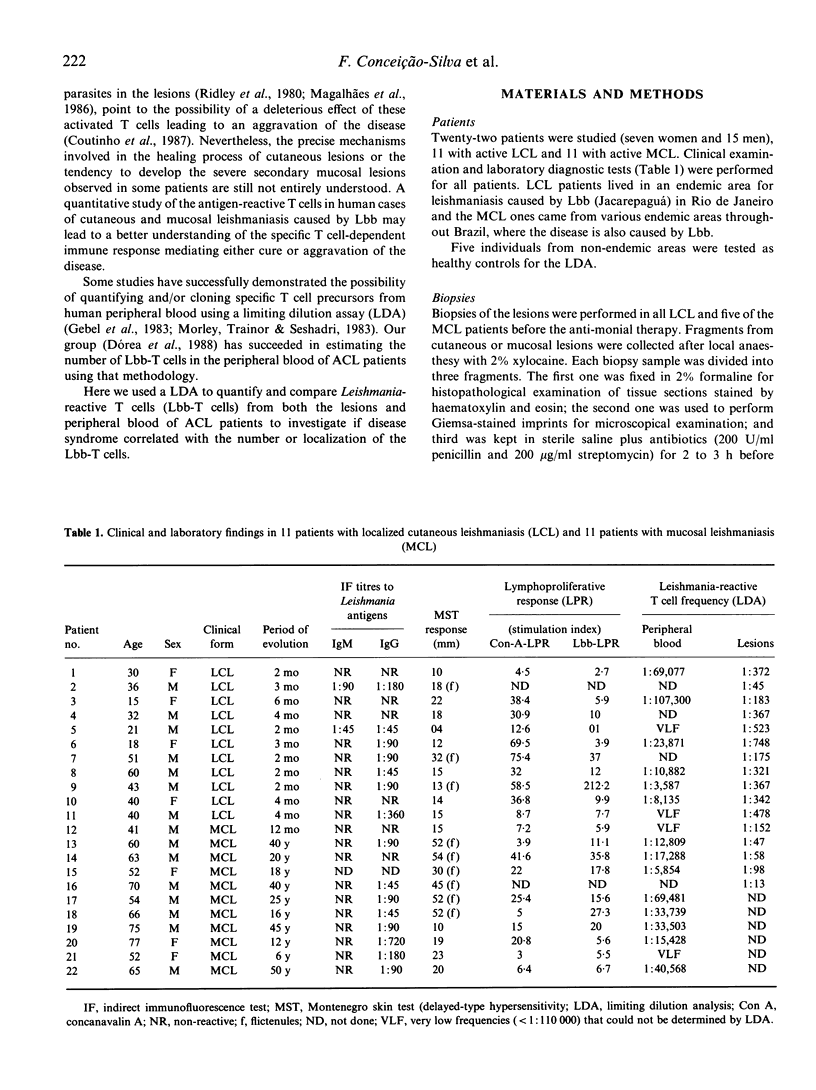
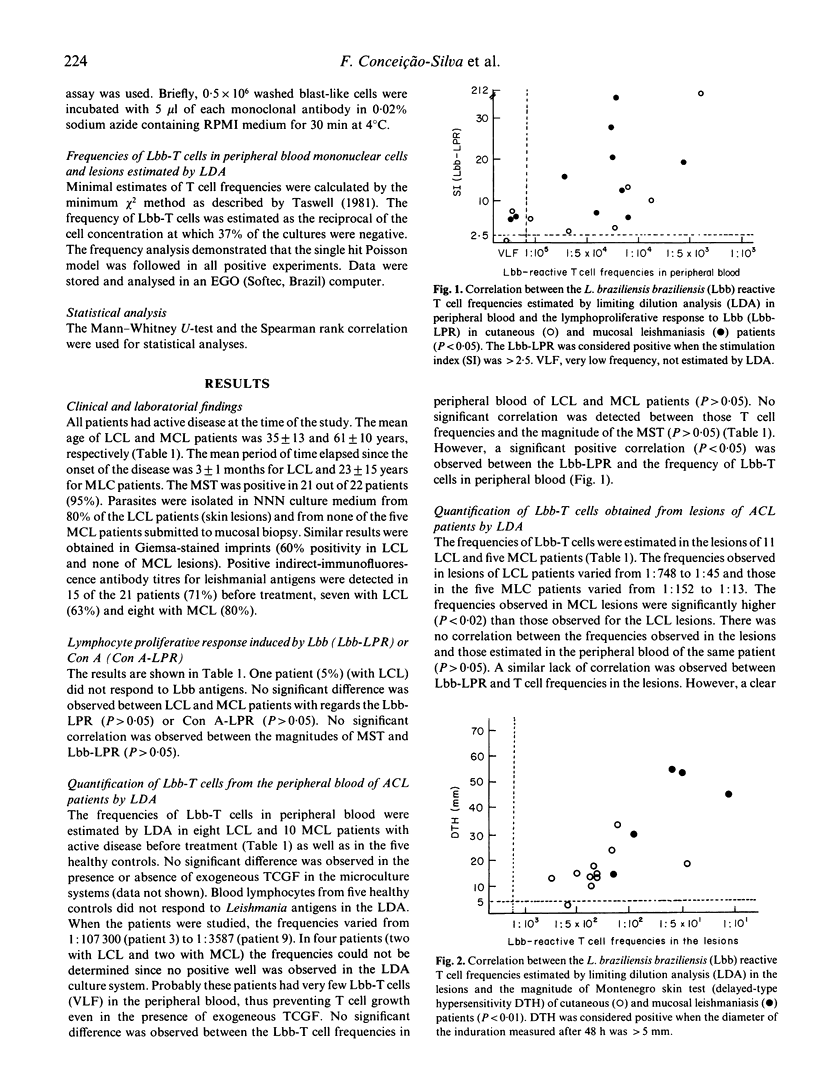
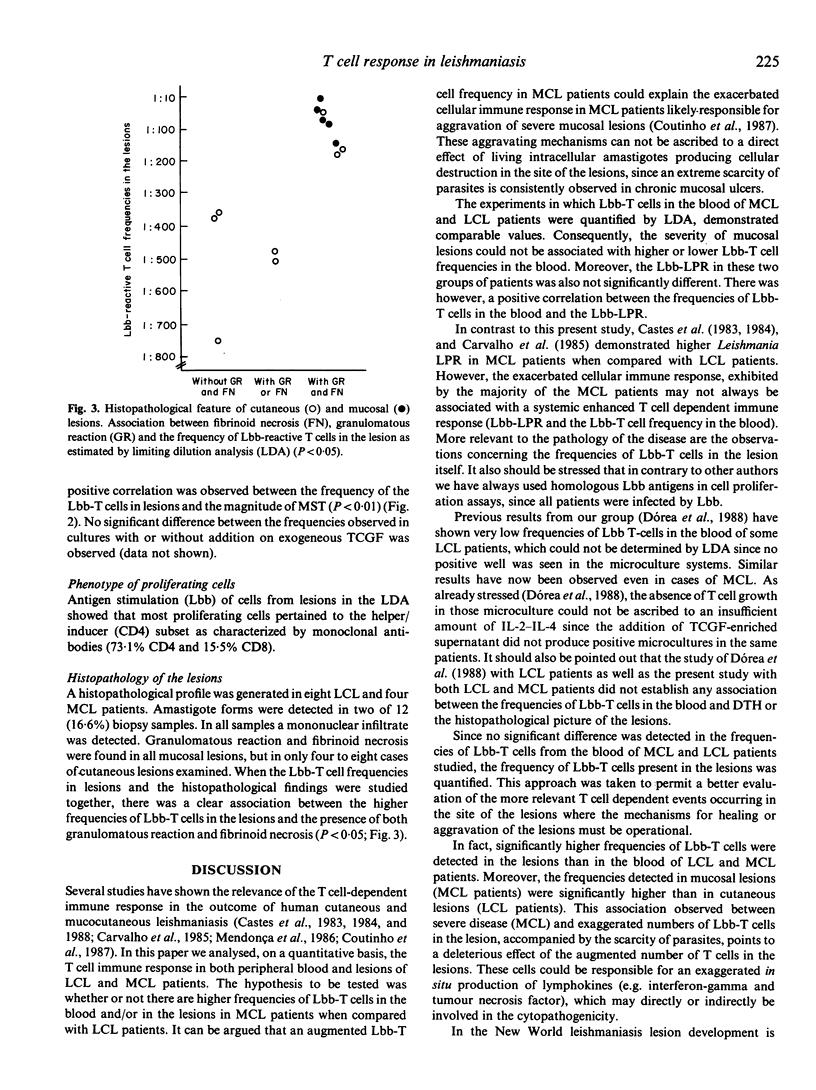
A limiting dilution analysis (LDA) was utilized to estimate the frequency of L. braziliensis braziliensis reactive T cells (Lbb-T cells) in peripheral blood and in the lesions of patients with mild localized cutaneous leishmaniasis (LCL) or with severe mucosal leishmaniasis (MCL). The frequencies of Lbb-T cells in peripheral blood varied from 1:107300 to 1:3587 and were not significantly different in MCL and LCL patients. However, a significant difference was encountered (P less than 0.02) between the T cells frequencies in cutaneous (1:748 to 1:45) and mucosal lesions (1:152 to 1:13). A positive correlation was also observed between these frequencies and the magnitude of delayed-type hypersensitivity (DTH) (P less than 0.01) and the presence of fibrinoid necrosis and granulomatous reaction in the site of the lesions (P less than 0.05). The lack of correlation between the severity of disease (MCL or LCL) and the frequency of Lbb-T cells in peripheral blood gave no indications towards understanding the physiopathology of severe or mild disease. However, the correlation between high T cell frequencies in the site of the lesions, the magnitude of DTH, the fibrinoid necrosis and the severity of the disease (MCL lesions) points to the possibility that the presence of a strong T cell dependent cellular immune response in the site of the lesions may have a deleterious effect. However, a local well modulated T cell immune response might provide healing of the lesions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Johnson W. D., Barreto E., Marsden P. D., Costa J. L., Reed S., Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985 Dec;135(6):4144–4148. [PubMed] [Google Scholar]
- Castes M., Agnelli A., Verde O., Rondón A. J. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol. 1983 May;27(2):176–186. doi: 10.1016/0090-1229(83)90068-5. [DOI] [PubMed] [Google Scholar]
- Castes M., Cabrera M., Trujillo D., Convit J. T-cell subpopulations, expression of interleukin-2 receptor, and production of interleukin-2 and gamma interferon in human American cutaneous leishmaniasis. J Clin Microbiol. 1988 Jun;26(6):1207–1213. doi: 10.1128/jcm.26.6.1207-1213.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castés M., Agnelli A., Rondón A. J. Mechanisms associated with immunoregulation in human American cutaneous leishmaniasis. Clin Exp Immunol. 1984 Aug;57(2):279–286. [PMC free article] [PubMed] [Google Scholar]
- Coutinho S. G., Louis J. A., Mauel J., Engers H. D. Induction by specific T lymphocytes of intracellular destruction of Leishmania major in infected murine macrophages. Parasite Immunol. 1984 Mar;6(2):157–169. doi: 10.1111/j.1365-3024.1984.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Gebel H. M., Scott J. R., Parvin C. A., Rodey G. E. In vitro immunization to KLH. II. Limiting dilution analysis of antigen-reactive cells in primary and secondary culture. J Immunol. 1983 Jan;130(1):29–32. [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainson R. The American leishmaniases: some observations on their ecology and epidemiology. Trans R Soc Trop Med Hyg. 1983;77(5):569–596. doi: 10.1016/0035-9203(83)90185-2. [DOI] [PubMed] [Google Scholar]
- Louis J. A., Zubler R. H., Coutinho S. G., Lima G., Behin R., Mauel J., Engers H. D. The in vitro generation and functional analysis of murine T cell populations and clones specific for a protozoan parasite, Leishmania tropica. Immunol Rev. 1982;61:215–243. doi: 10.1111/j.1600-065x.1982.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Mendonça S. C., Coutinho S. G., Amendoeira R. R., Marzochi M. C., Pirmez C. Human american cutaneous leishmaniasis (Leishmania b. braziliensis) in Brazil: lymphoproliferative responses and influence of therapy. Clin Exp Immunol. 1986 May;64(2):269–276. [PMC free article] [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R. S. Cloning of human lymphocytes using limiting dilution. Exp Hematol. 1983 May;11(5):418–424. [PubMed] [Google Scholar]
- Ridley D. S., Marsden P. D., Cuba C. C., Barreto A. C. A histological classification of mucocutaneous leishmaniasis in Brazil and its clinical evaluation. Trans R Soc Trop Med Hyg. 1980;74(4):508–514. doi: 10.1016/0035-9203(80)90068-1. [DOI] [PubMed] [Google Scholar]
- Ridley D. S. The pathogenesis of cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73(2):150–160. doi: 10.1016/0035-9203(79)90199-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Mendonça-Previato L., Charlab R., Barcinski M. A. The cellular immune response to a purified antigen from Leishmania mexicana subsp. amazonensis enhances the size of the leishmanial lesion on susceptible mice. Infect Immun. 1987 Dec;55(12):3142–3148. doi: 10.1128/iai.55.12.3142-3148.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart Y. C., Cox J., Roberts T. K., Brinsmead M. W., Burton R. C. Differential effect of cigarette smoking on recirculating T lymphocyte subsets in pregnant women. J Immunol. 1986 Jul 1;137(1):1–3. [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]


