Abstract
Helicobacter pylori is a major cause of chronic antral gastritis and peptic ulcer disease. Further definition is needed of the factors that determine whether infected individuals remain asymptomatic, or ultimately develop ulceration of the mucosa or transformation to malignancy. To explore the possibility that host response to H. pylori may play a role in the outcome of this infection, we have examined humoral and cellular recognition of several H. pylori proteins by seropositive and seronegative persons. A complex mixture of water-extractable cell proteins, which did not include lipopolysaccharide (LPS), was recognized by serum antibodies only in seropositive or infected individuals. IgG from seropositive subjects also bound to urease and to a heat shock protein (hsp)60 that is homologous to the 65-kD mycobacterial heat shock protein, while sera from uninfected individuals were negative. Although antibody responses to these antigens were restricted to seropositive subjects, T cell recognition of the same proteins was found in both seropositive and seronegative subjects. The water extract of H. pylori stimulated peripheral blood mononuclear cells (PBMC) from all subjects, while purified proteins activated lymphocytes of only some seropositive and seronegative subjects. PBMC that were activated by the H. pylori hsp60 did not respond to the autologous human p60 heat shock protein. These results demonstrate that, in contrast to antibody responses, T cell recognition of H. pylori proteins may occur in non-infected persons. In addition, the data suggest that in these subjects, peripheral lymphocytes that are activated by bacterial heat shock proteins do not mediate tissue damage by recognition of human heat shock homologues.
Full text
PDF
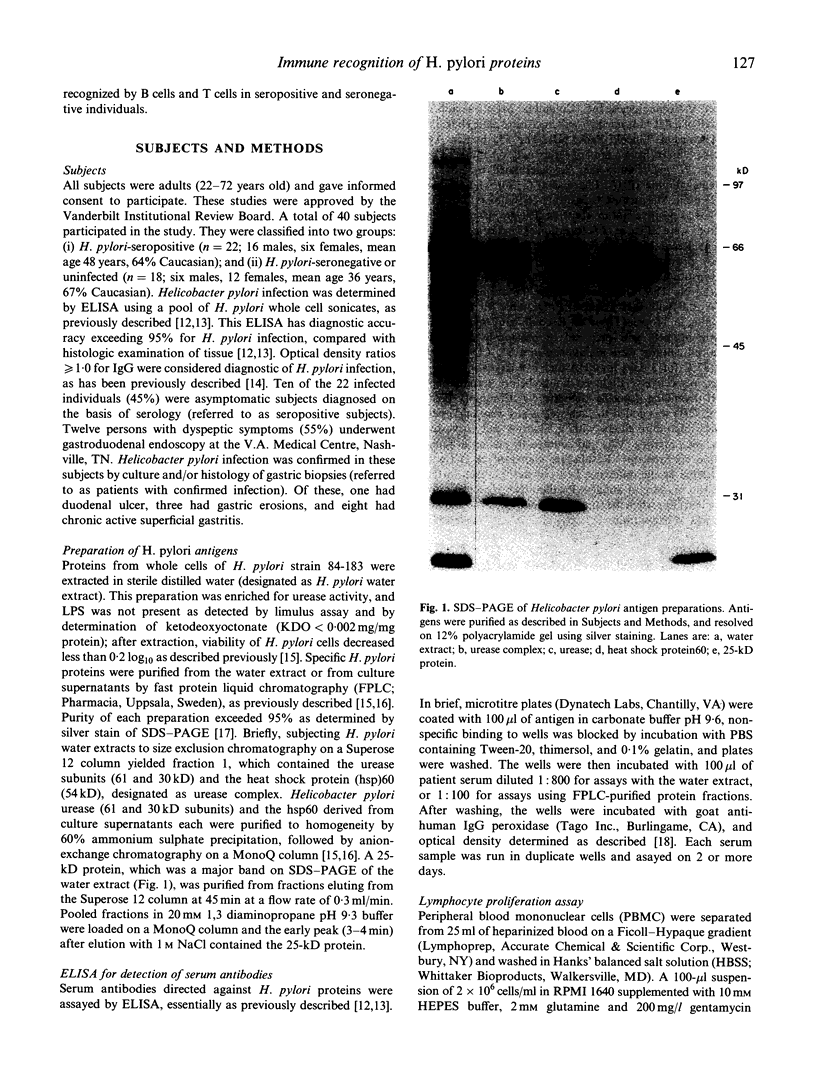

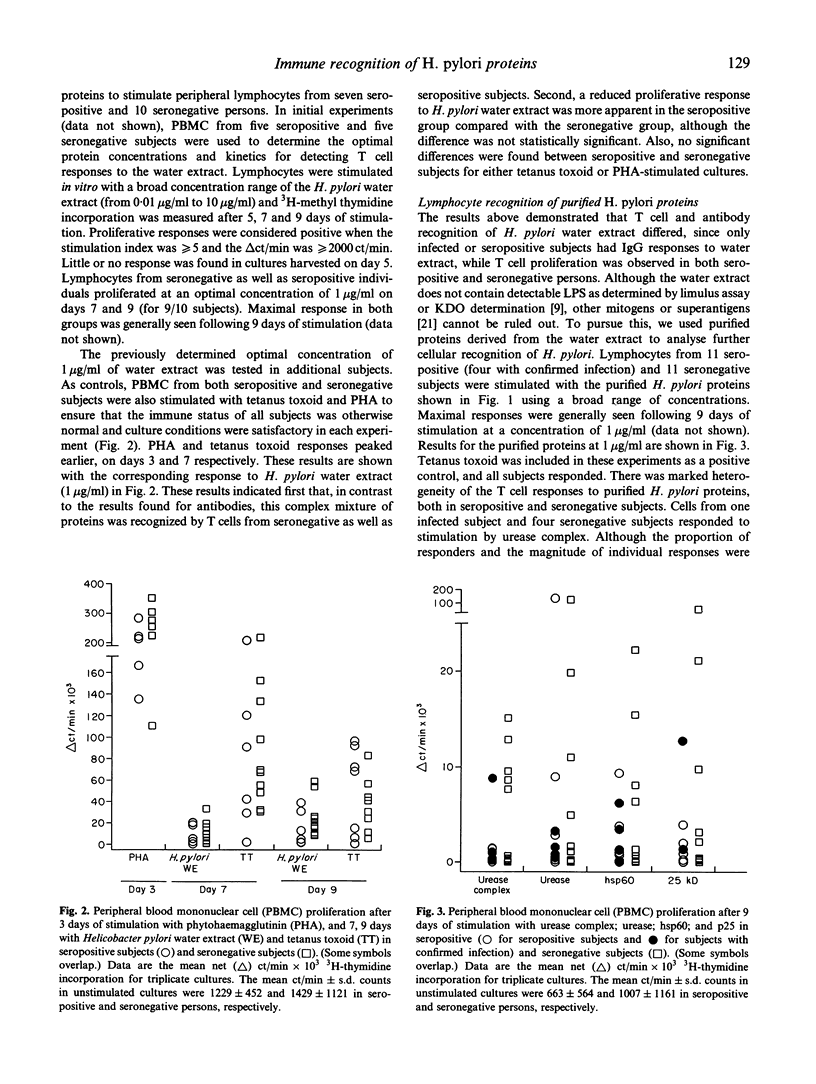
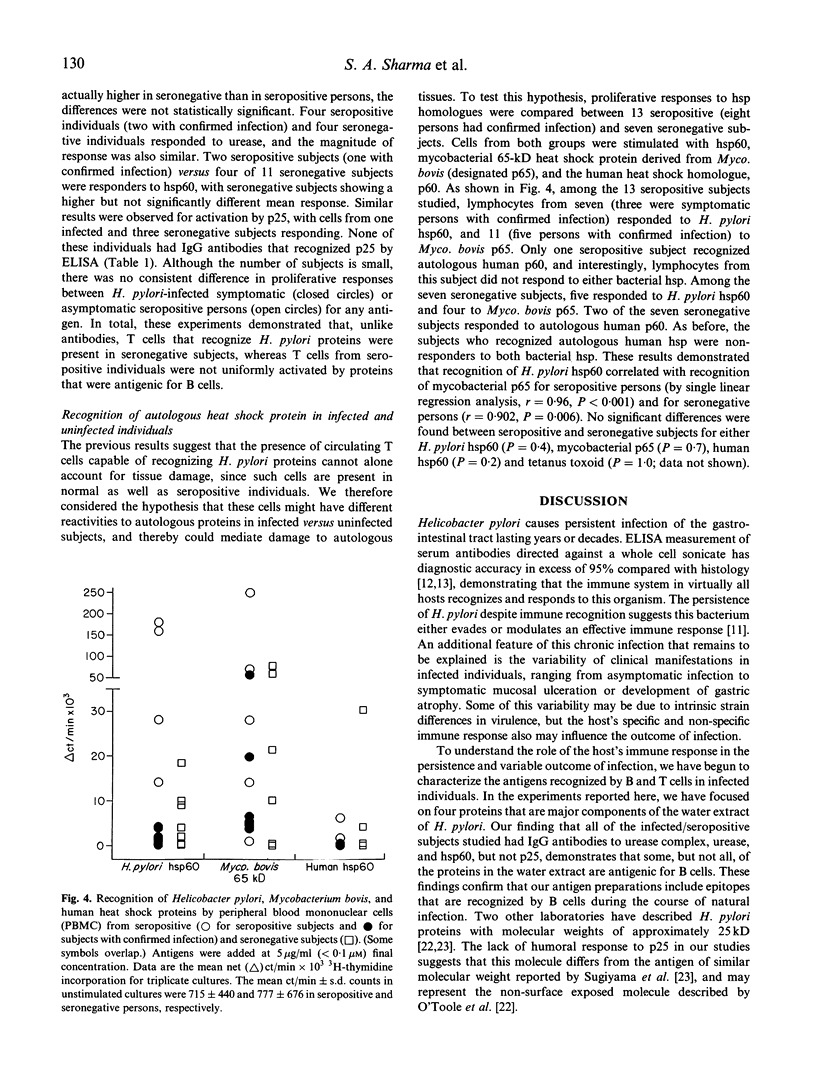


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkholz S., Knipp U., Opferkuch W. Stimulatory effects of Helicobacter pylori on human peripheral blood mononuclear cells of H. pylori infected patients and healthy blood donors. Zentralbl Bakteriol. 1993 Sep;280(1-2):166–176. doi: 10.1016/s0934-8840(11)80953-9. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992 Feb;102(2):720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- Caselli M., Alvisi V. Bacterial adhesion and Helicobacter pylori. Gut. 1991 Jan;32(1):111–112. doi: 10.1136/gut.32.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley C. P., Cohen H., Fitzgibbons P. L., Bauer M., Appleman M. D., Perez-Perez G. I., Blaser M. J. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989 Dec 7;321(23):1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- Drumm B., Perez-Perez G. I., Blaser M. J., Sherman P. M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990 Feb 8;322(6):359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990 Jun 5;265(16):9464–9469. [PubMed] [Google Scholar]
- Dunn B. E., Perez-Perez G. I., Blaser M. J. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter pylori proteins. Infect Immun. 1989 Jun;57(6):1825–1833. doi: 10.1128/iai.57.6.1825-1833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Roop R. M., 2nd, Sung C. C., Sharma S. A., Perez-Perez G. I., Blaser M. J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992 May;60(5):1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Reshef T., Birk O. S., van der Zee R., Walker M. D., Cohen I. R. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrand L., Scheynius A., Påhlson C. An increased number of gamma/delta T-cells and gastric epithelial cell expression of the groEL stress-protein homologue in Helicobacter pylori-associated chronic gastritis of the antrum. Am J Gastroenterol. 1991 Aug;86(8):976–980. [PubMed] [Google Scholar]
- Karttunen R., Andersson G., Poikonen K., Kosunen T. U., Karttunen T., Juutinen K., Niemelä S. Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin Exp Immunol. 1990 Dec;82(3):485–488. doi: 10.1111/j.1365-2249.1990.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen R. Blood lymphocyte proliferation, cytokine secretion and appearance of T cells with activation surface markers in cultures with Helicobacter pylori. Comparison of the responses of subjects with and without antibodies to H. pylori. Clin Exp Immunol. 1991 Mar;83(3):396–400. doi: 10.1111/j.1365-2249.1991.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Macchia G., Massone A., Burroni D., Covacci A., Censini S., Rappuoli R. The Hsp60 protein of Helicobacter pylori: structure and immune response in patients with gastroduodenal diseases. Mol Microbiol. 1993 Aug;9(3):645–652. doi: 10.1111/j.1365-2958.1993.tb01724.x. [DOI] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Allen J. B., Wahl S. M., Blaser M. J., Smith P. D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992 Feb 1;175(2):517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Wahl L. M., Wahl S. M., Blaser M. J., Smith P. D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991 Mar;87(3):894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollick J. A., Miller G. G., Musser J. M., Cook R. G., Grossman D., Rich R. R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with aminoterminal homology to staphylococcal enterotoxins B and C. J Clin Invest. 1993 Aug;92(2):710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Logan S. M., Kostrzynska M., Wadström T., Trust T. J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991 Jan;173(2):505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Blaser M. J., Perez-Perez G. I., Hargrett-Bean N., Tauxe R. V. Symptoms and risk factors of Helicobacter pylori infection in a cohort of epidemiologists. Gastroenterology. 1992 Jan;102(1):41–46. doi: 10.1016/0016-5085(92)91782-y. [DOI] [PubMed] [Google Scholar]
- Perez-Perez G. I., Blaser M. J. Conservation and diversity of Campylobacter pyloridis major antigens. Infect Immun. 1987 May;55(5):1256–1263. doi: 10.1128/iai.55.5.1256-1263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Dworkin B. M., Chodos J. E., Blaser M. J. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988 Jul 1;109(1):11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- Peterson W. L. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991 Apr 11;324(15):1043–1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- Pope R. M., Lovis R. M., Gupta R. S. Activation of synovial fluid T lymphocytes by 60-kd heat-shock proteins in patients with inflammatory synovitis. Arthritis Rheum. 1992 Jan;35(1):43–48. doi: 10.1002/art.1780350107. [DOI] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Roche P. W., Theuvenet W. J., Britton W. J. Cellular immune responses to mycobacterial heat shock proteins in Nepali leprosy patients and controls. Int J Lepr Other Mycobact Dis. 1992 Mar;60(1):36–43. [PubMed] [Google Scholar]
- Singh B., Gupta R. S. Expression of human 60-kD heat shock protein (HSP60 or P1) in Escherichia coli and the development and characterization of corresponding monoclonal antibodies. DNA Cell Biol. 1992 Jul-Aug;11(6):489–496. doi: 10.1089/dna.1992.11.489. [DOI] [PubMed] [Google Scholar]
- Strauss R. M., Wang T. C., Kelsey P. B., Compton C. C., Ferraro M. J., Perez-Perez G., Parsonnet J., Blaser M. J. Association of Helicobacter pylori infection with dyspeptic symptoms in patients undergoing gastroduodenoscopy. Am J Med. 1990 Oct;89(4):464–469. doi: 10.1016/0002-9343(90)90377-p. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Imai K., Yoshida H., Takayama Y., Yabana T., Yokota K., Oguma K., Yachi A. A novel enzyme immunoassay for serodiagnosis of Helicobacter pylori infection. Gastroenterology. 1991 Jul;101(1):77–83. doi: 10.1016/0016-5085(91)90462-t. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Calabrese A., Smart C. J., Oakes D. J., Howdle P. D., Crabtree J. E., Losowsky M. S., Lancaster F., Boylston A. W. Gamma delta T cell receptor-positive cells of the human gastrointestinal mucosa: occurrence and V region gene expression in Heliobacter pylori-associated gastritis, coeliac disease and inflammatory bowel disease. Clin Exp Immunol. 1991 Jun;84(3):440–444. [PMC free article] [PubMed] [Google Scholar]