Abstract
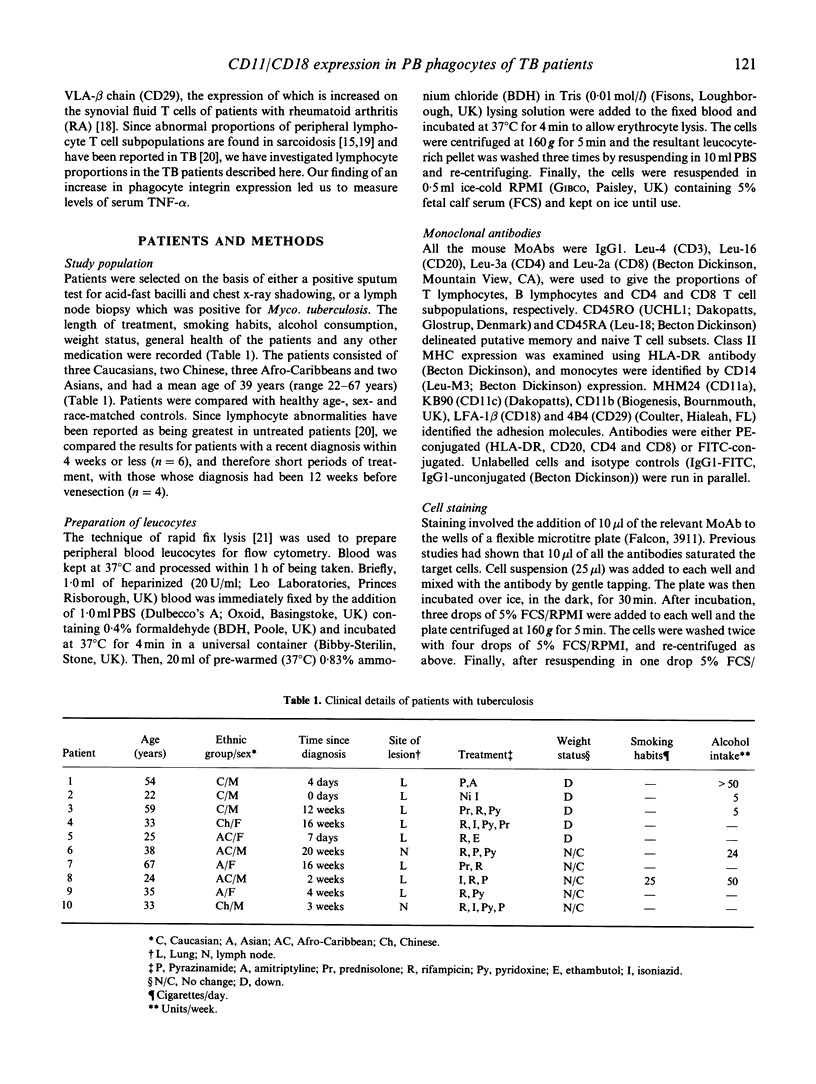
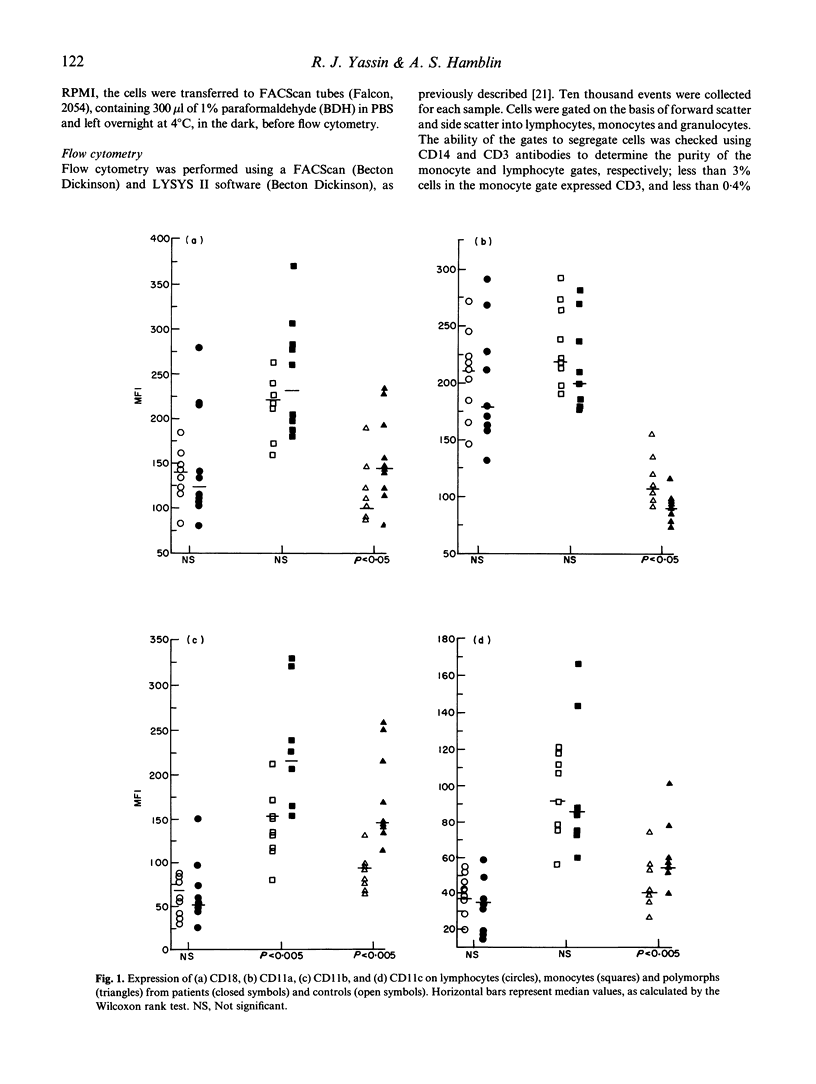
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is characterized by granulomatous lesions made up of epithelioid cells, giant cells and mononuclear leucocytes. Cell-cell adhesion is important in granuloma formation and in the leucocyte migration which accompanies it. We have recently shown increased expression of the adhesion molecules CD11/CD18 (LeuCAMs, beta 2 integrins) on peripheral blood leucocytes from patients with sarcoidosis (Shakoor & Hamblin, 1992). Here we have studied the expression of CD11/CD18 and CD29 (VLA beta 1 integrin) on the peripheral blood leucocytes of 10 TB patients by flow cytometry. The density (expressed as mean fluorescence intensity) of CD11b on monocytes and polymorphs was increased (P < 0.005), as was CD11c (P < 0.005) and CD18 (P < 0.05) on polymorphs. CD11a expression was significantly reduced on polymorphs (P < 0.05). No differences were found in the expression of CD29, the percentages of cells expressing any molecule and, in contrast to sarcoidosis, the density of any molecule on lymphocytes. Although the cytokine tumour necrosis factor (TNF) has been implicated in the process of up-regulation, an ELISA for TNF failed to detect significant levels in plasma. The results suggest increased peripheral phagocyte CD11/CD18 expression is a feature of TB, which may contribute to the pathological processes involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainslie G. M., Solomon J. A., Bateman E. D. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax. 1992 Jul;47(7):513–518. doi: 10.1136/thx.47.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Appelberg R. T cell regulation of the chronic peritoneal neutrophilia during mycobacterial infections. Clin Exp Immunol. 1992 Jul;89(1):120–125. doi: 10.1111/j.1365-2249.1992.tb06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Hakim R. M., Todd R. F., 3rd, Dana N., Colten H. R. Increased expression of an adhesion-promoting surface glycoprotein in the granulocytopenia of hemodialysis. N Engl J Med. 1985 Feb 21;312(8):457–462. doi: 10.1056/NEJM198502213120801. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A. Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990 Apr;114:145–180. doi: 10.1111/j.1600-065x.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Bocart D., Lecossier D., De Lassence A., Valeyre D., Battesti J. P., Hance A. J. A search for mycobacterial DNA in granulomatous tissues from patients with sarcoidosis using the polymerase chain reaction. Am Rev Respir Dis. 1992 May;145(5):1142–1148. doi: 10.1164/ajrccm/145.5.1142. [DOI] [PubMed] [Google Scholar]
- Cadranel J., Philippe C., Philippe B., Milleron B., Fouqueray B., Mayaud C., Baud L. Increased expression and occupancy of receptors for tumour necrosis factor on blood monocytes from tuberculosis patients. Clin Exp Immunol. 1993 Oct;94(1):51–56. doi: 10.1111/j.1365-2249.1993.tb05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991 Jul;12(7):228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J., Schachter B. Z. Augmentation of selective monocyte functions in tuberculosis. J Infect Dis. 1981 Nov;144(5):391–398. doi: 10.1093/infdis/144.5.391. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Kleinhenz M. E., Wallis R. S., Ellner J. J. Increased interleukin-1 production and monocyte suppressor cell activity associated with human tuberculosis. Am Rev Respir Dis. 1986 Jan;133(1):73–77. doi: 10.1164/arrd.1986.133.1.73. [DOI] [PubMed] [Google Scholar]
- Greenfield S. M., Hamblin A., Punchard N. A., Thompson R. P. Expression of adhesion molecules on circulating leucocytes in patients with inflammatory bowel disease. Clin Sci (Lond) 1992 Aug;83(2):221–226. doi: 10.1042/cs0830221. [DOI] [PubMed] [Google Scholar]
- Hamblin A. S., Shakoor Z., Kapahi P., Haskard D. Circulating adhesion molecules in sarcoidosis. Clin Exp Immunol. 1994 May;96(2):335–338. doi: 10.1111/j.1365-2249.1994.tb06563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin A., Taylor M., Bernhagen J., Shakoor Z., Mayall S., Noble G., McCarthy D. A method of preparing blood leucocytes for flow cytometry which prevents upregulation of leucocyte integrins. J Immunol Methods. 1992 Feb 5;146(2):219–228. doi: 10.1016/0022-1759(92)90231-h. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Kuratsuji T., Tsunawaki S., Aizawa S., Toyama K. In vivo effects of recombinant human granulocyte colony-stimulating factor on normal neutrophil function and membrane effector molecule expression. Int J Hematol. 1991 Dec;54(6):463–469. [PubMed] [Google Scholar]
- Laffón A., García-Vicuña R., Humbría A., Postigo A. A., Corbí A. L., de Landázuri M. O., Sánchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991 Aug;88(2):546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. K., Wong K. C., Chan C. H., Ho S. S., Chung S. Y., Haskard D. O., Lai K. N. Circulating adhesion molecules in tuberculosis. Clin Exp Immunol. 1993 Dec;94(3):522–526. [PMC free article] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Hamblin A. S., Wolstencroft R. A., Dumonde D. C. Rapid cytokine up-regulation of integrins, complement receptor 1 and HLA-DR on monocytes but not on lymphocytes. Immunology. 1992 Sep;77(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- Limb G. A., Hamblin A. S., Wolstencroft R. A., Dumonde D. C. Selective up-regulation of human granulocyte integrins and complement receptor 1 by cytokines. Immunology. 1991 Dec;74(4):696–702. [PMC free article] [PubMed] [Google Scholar]
- McCarthy D., Taylor M. J., Bernhagen J., Perry J. D., Hamblin A. S. Leucocyte integrin and CR1 expression on peripheral blood leucocytes of patients with rheumatoid arthritis. Ann Rheum Dis. 1992 Mar;51(3):307–312. doi: 10.1136/ard.51.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Rees A. J. Striking the right balance; the role of cytokines in mycobacterial disease. Clin Exp Immunol. 1993 Oct;94(1):1–3. doi: 10.1111/j.1365-2249.1993.tb05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Ozaki T., Nakahira S., Tani K., Ogushi F., Yasuoka S., Ogura T. Differential cell analysis in bronchoalveolar lavage fluid from pulmonary lesions of patients with tuberculosis. Chest. 1992 Jul;102(1):54–59. doi: 10.1378/chest.102.1.54. [DOI] [PubMed] [Google Scholar]
- Palmer S., Hamblin A. S. Increased CD11/CD18 expression on the peripheral blood leucocytes of patients with HIV disease: relationship to disease severity. Clin Exp Immunol. 1993 Sep;93(3):344–349. doi: 10.1111/j.1365-2249.1993.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn L. J., Laufer E. M., Land H. C-MYC: evidence for multiple regulatory functions. Semin Cancer Biol. 1990 Feb;1(1):69–80. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Monocyte IgG receptor activity, dynamics, and modulation-normal individuals and patients with granulomatous diseases. J Lab Clin Med. 1977 Feb;89(2):332–340. [PubMed] [Google Scholar]
- Shakoor Z., Hamblin A. S. Increased CD11/CD18 expression on peripheral blood leucocytes of patients with sarcoidosis. Clin Exp Immunol. 1992 Oct;90(1):99–105. doi: 10.1111/j.1365-2249.1992.tb05839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shijubo N., Imai K., Nakanishi F., Yachi A., Abe S. Elevated concentrations of circulating ICAM-1 in far advanced and miliary tuberculosis. Am Rev Respir Dis. 1993 Nov;148(5):1298–1301. doi: 10.1164/ajrccm/148.5.1298. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Beekhuizen H., van Furth R. Surface molecules involved in the adherence of recombinant interferon-gamma (rIFN-gamma)-stimulated human monocytes to vascular endothelial cells. Clin Exp Immunol. 1994 Feb;95(2):263–269. doi: 10.1111/j.1365-2249.1994.tb06521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Hellewell P. G. Endothelial cell biology. Adhesion molecules involved in the microvascular inflammatory response. Am Rev Respir Dis. 1992 Nov;146(5 Pt 2):S45–S50. doi: 10.1164/ajrccm/146.5_Pt_2.S45. [DOI] [PubMed] [Google Scholar]
- van de Wiel-van Kemenade E., van Kooyk Y., de Boer A. J., Huijbens R. J., Weder P., van de Kasteele W., Melief C. J., Figdor C. G. Adhesion of T and B lymphocytes to extracellular matrix and endothelial cells can be regulated through the beta subunit of VLA. J Cell Biol. 1992 Apr;117(2):461–470. doi: 10.1083/jcb.117.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


