Abstract
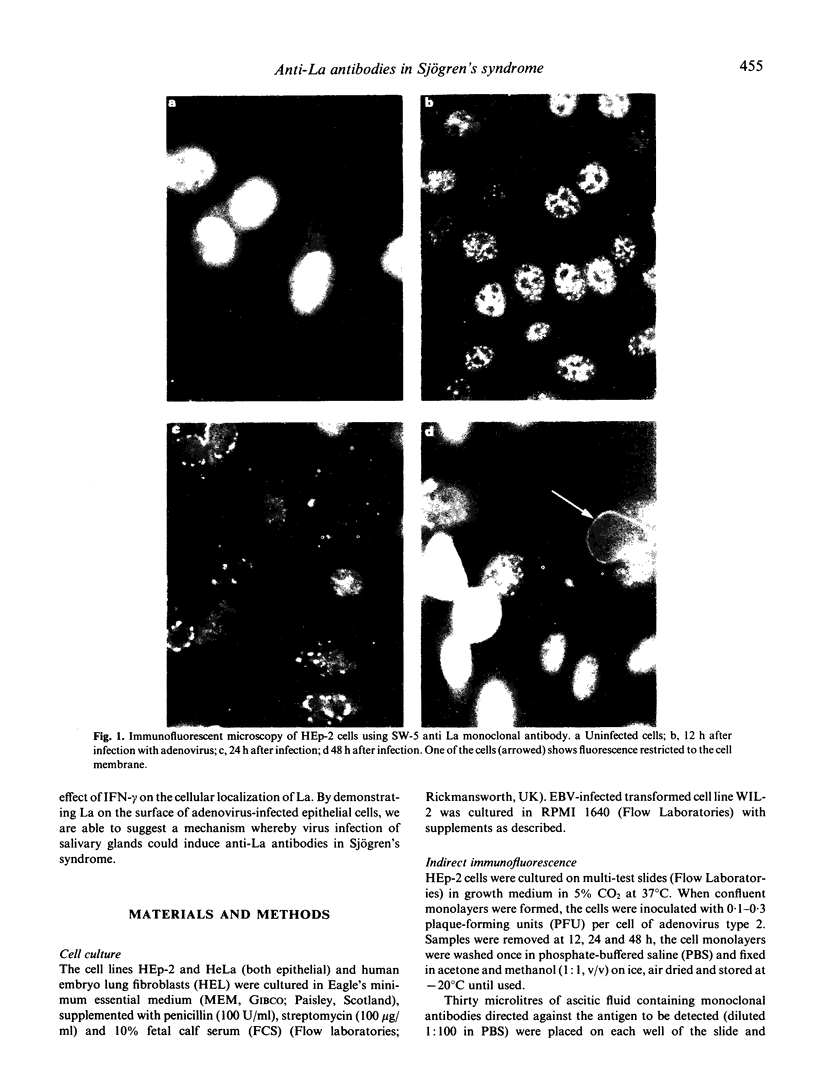
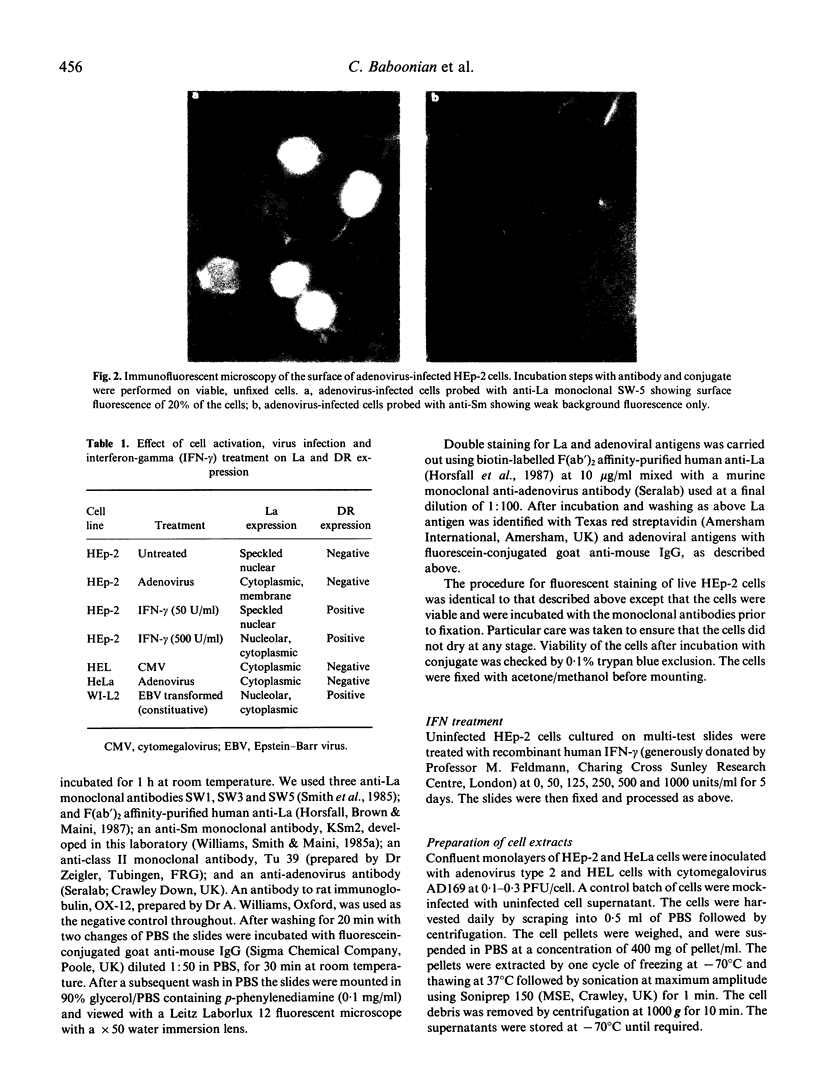
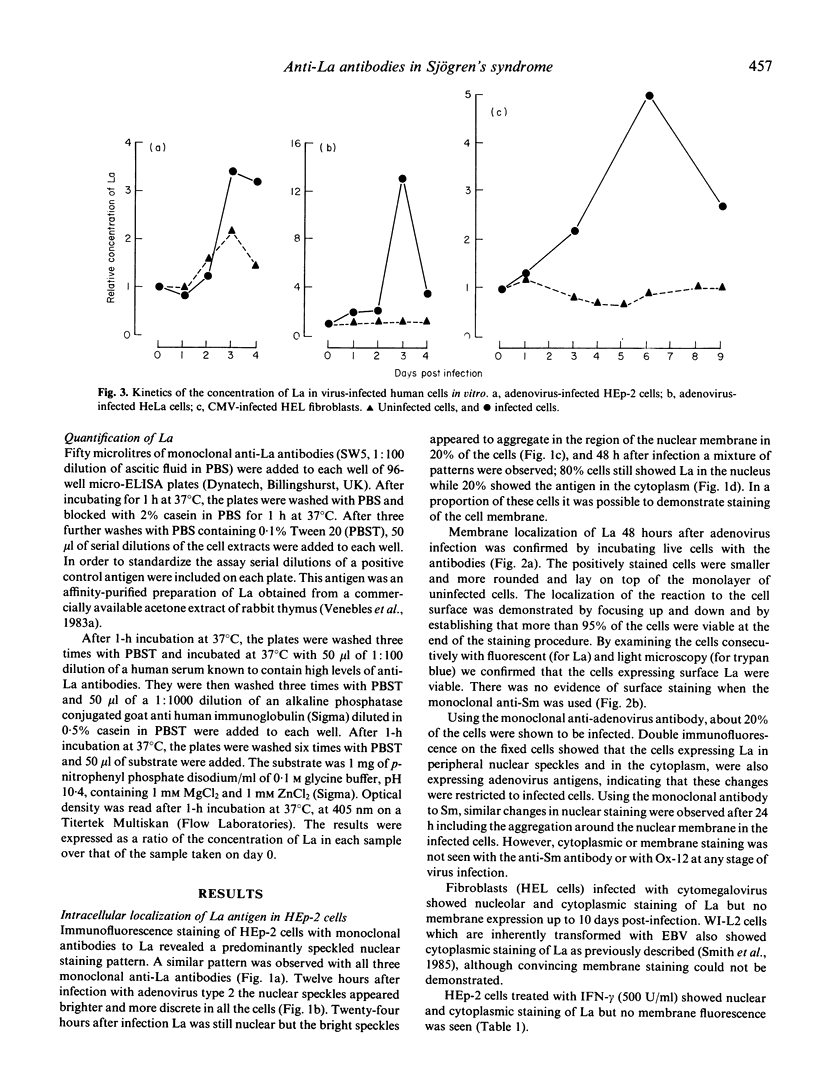
To investigate the possibility that anti-La (SS-B) antibodies in Sjögren's syndrome were induced by virus infection we studied the distribution of La in virus-infected human cell lines. Three monoclonal antibodies to La were used with monoclonal anti-Sm (derived from MRL/lpr lupus mice) and anti-rat immunoglobulin antibodies as controls. In uninfected cells La was predominantly in the nucleus. Twenty-four hours after infection of HEp-2 cells with adenovirus 2, the La and Sm antigens appeared to aggregate and accumulate in the periphery of the nucleus and, after 48 h, La was seen in the cytoplasm and cell membrane. No cytoplasmic or membrane expression of Sm was seen. Infection with adenovirus or cytomegalovirus caused a 2-13-fold increase in the concentration of La in three cell lines. Treatment of HE--2 cells with interferon-gamma (IFN-gamma) and infection with Epstein-Barr virus and cytomegalovirus caused cytoplasmic, but no definite membrane expression of La. The appearance of La on the surface of virally infected epithelial cells together with IFN-gamma induced class II expression could form the basis of a T cell dependent mechanism for anti-La autoantibody induction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alspaugh M. A., Talal N., Tan E. M. Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum. 1976 Mar-Apr;19(2):216–222. doi: 10.1002/art.1780190214. [DOI] [PubMed] [Google Scholar]
- Bachmann M., Falke D., Preuhs J., Schröder H. C., Pfeifer K., Müller W. E. Occurrence of novel small RNAs with concomitant inhibition of host cellular U small nuclear RNA synthesis in Vero cells infected with herpes simplex virus type 1. J Gen Virol. 1986 Dec;67(Pt 12):2587–2594. doi: 10.1099/0022-1317-67-12-2587. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Bumol T., Fantozzi R., Bone R., Schreiber R. Expression of histocompatibility antigen HLA-DR by salivary gland epithelial cells in Sjögren's syndrome. Arthritis Rheum. 1986 Sep;29(9):1105–1111. doi: 10.1002/art.1780290908. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., den Brok J. H., Boerbooms A. M., van de Putte L. B., van Venrooij W. J. Characterization of the SS-B (La) antigen in adenovirus-infected and uninfected HeLa cells. EMBO J. 1983;2(10):1625–1631. doi: 10.1002/j.1460-2075.1983.tb01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfall A. C., Brown C. M., Maini R. N. Purification of human autoantibodies from cross-linked antigen immunosorbents. J Immunol Methods. 1987 Nov 23;104(1-2):43–49. doi: 10.1016/0022-1759(87)90485-6. [DOI] [PubMed] [Google Scholar]
- LeFeber W. P., Norris D. A., Ryan S. R., Huff J. C., Lee L. A., Kubo M., Boyce S. T., Kotzin B. L., Weston W. L. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984 Oct;74(4):1545–1551. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Hedfors E., Klareskog L., Forsum U. Epithelial HLA-DR expression and T lymphocyte subsets in salivary glands in Sjögren's syndrome. Clin Exp Immunol. 1985 Sep;61(3):475–482. [PMC free article] [PubMed] [Google Scholar]
- Martin T. E., Barghusen S. C., Leser G. P., Spear P. G. Redistribution of nuclear ribonucleoprotein antigens during herpes simplex virus infection. J Cell Biol. 1987 Nov;105(5):2069–2082. doi: 10.1083/jcb.105.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum. 1974 Jul-Aug;17(4):421–429. doi: 10.1002/art.1780170413. [DOI] [PubMed] [Google Scholar]
- Phillips P. E. Evidence implicating infectious agents in rheumatoid arthritis and juvenile rheumatoid arthritis. Clin Exp Rheumatol. 1988 Jan-Mar;6(1):87–94. [PubMed] [Google Scholar]
- Pizer L. I., Deng J. S., Stenberg R. M., Tan E. M. Characterization of a phosphoprotein associated with the SS-B/La nuclear antigen in adenovirus-infected and uninfected KB cells. Mol Cell Biol. 1983 Jul;3(7):1235–1245. doi: 10.1128/mcb.3.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. R., Williams D. G., Venables P. J., Maini R. N. Monoclonal antibodies to the Sjögren's syndrome associated antigen SS-B (La). J Immunol Methods. 1985 Feb 28;77(1):63–76. doi: 10.1016/0022-1759(85)90184-x. [DOI] [PubMed] [Google Scholar]
- Venables P. J., Charles P. J., Buchanan R. R., Yi T., Mumford P. A., Schrieber L., Room G. R., Maini R. N. Quantitation and detection of isotypes of anti-SS-B antibodies by ELISA and Farr assays using affinity purified antigens. An approach to the investigation of Sjögren's syndrome and systemic lupus erythematosus. Arthritis Rheum. 1983 Feb;26(2):146–155. doi: 10.1002/art.1780260205. [DOI] [PubMed] [Google Scholar]
- Venables P. J., Smith P. R., Maini R. N. Purification and characterization of the Sjögren's syndrome A and B antigens. Clin Exp Immunol. 1983 Dec;54(3):731–738. [PMC free article] [PubMed] [Google Scholar]


