Abstract
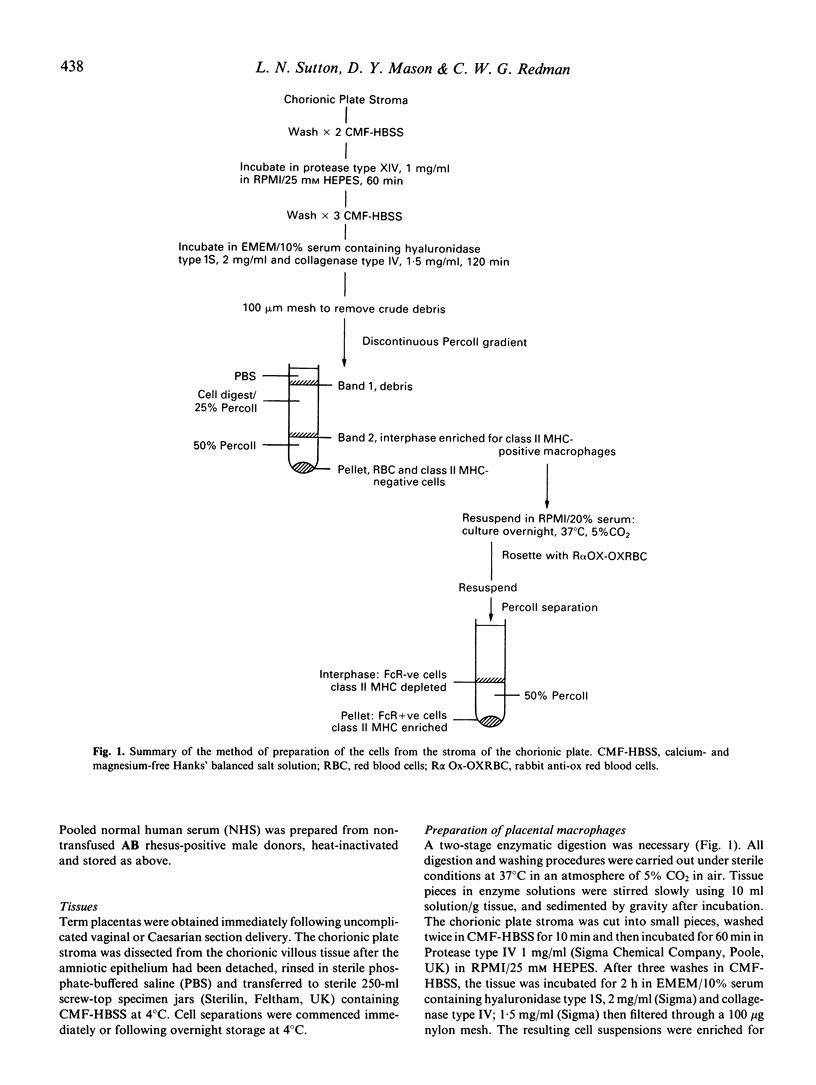
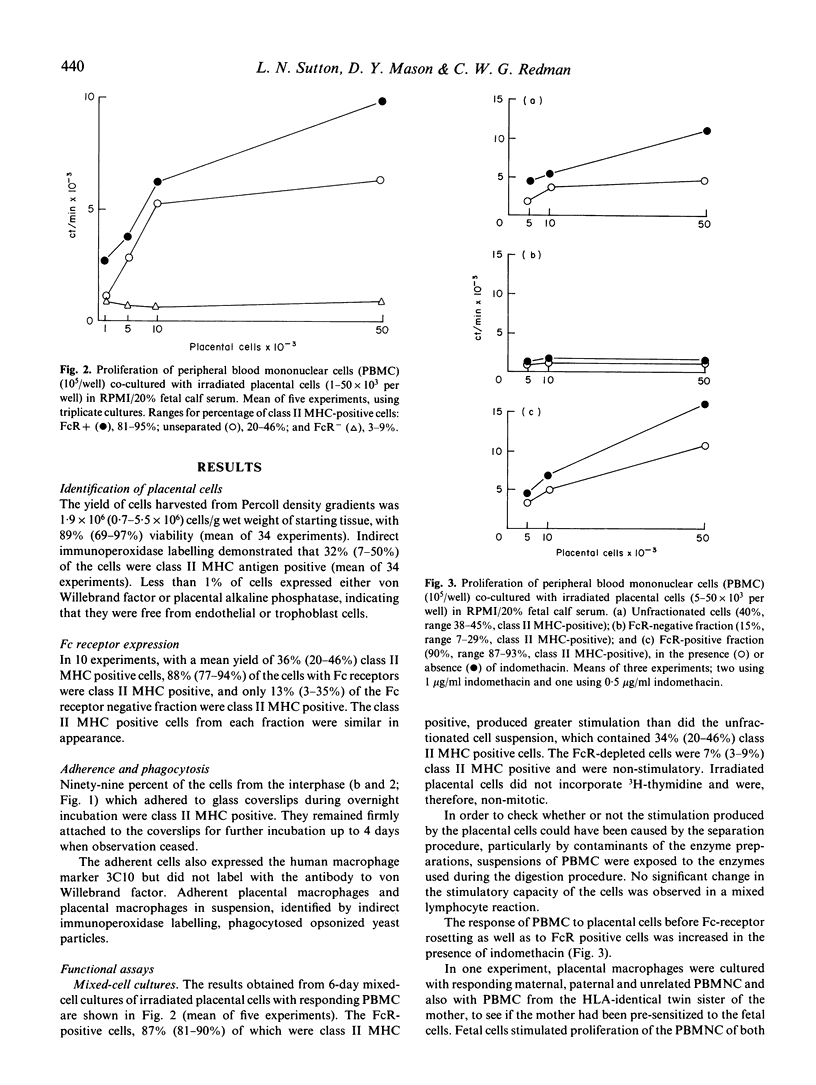
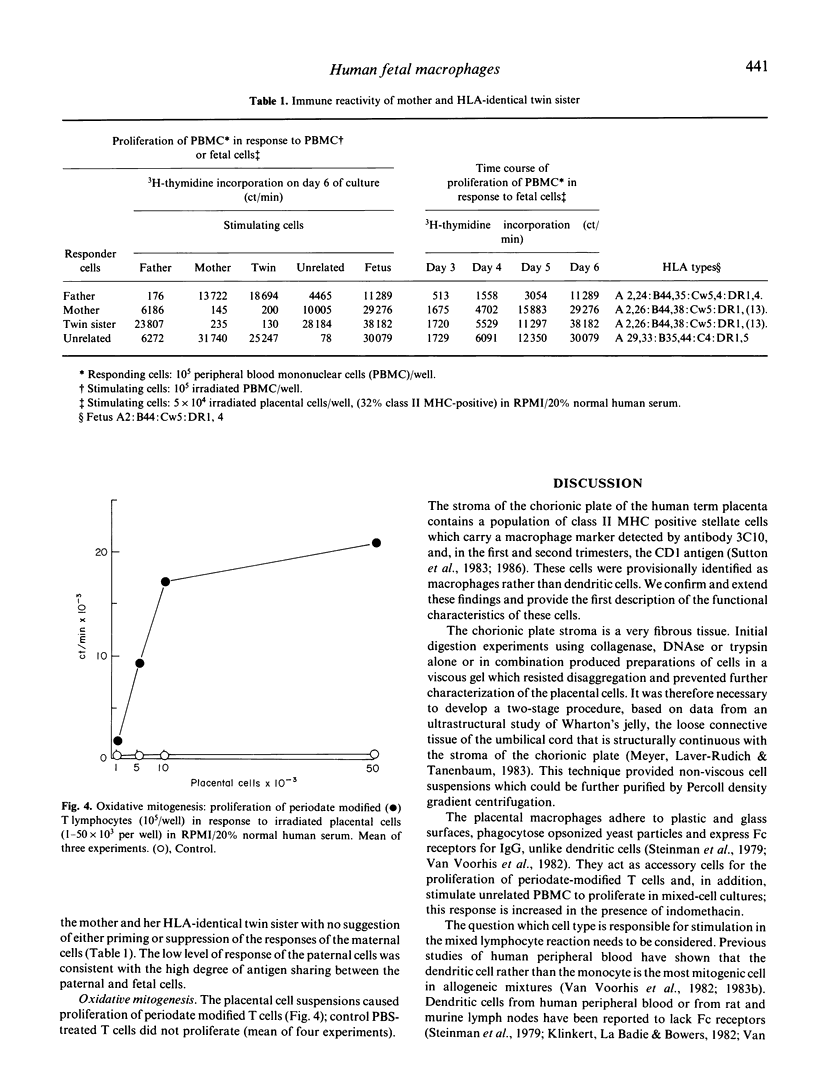
Human fetal macrophages expressing class II major histocompatibility complex (MHC) antigens have been isolated from the stroma of the chorionic plate of term placentas, using enzymatic digestion procedures, and enriched by Percoll density centrifugation. These cells are adherent, phagocytic and express Fc receptors for IgG. By rosetting with bovine erythrocytes coated with IgG, they can be enriched to 77-95% purity. Placental macrophages isolated in this way stimulate the proliferation of lymphocytes from unrelated donors in mixed-cell cultures, and act as accessory cells in oxidative mitogenesis. In a family study, placental macrophages stimulated proliferation of maternal and paternal lymphocytes but there was no evidence for either priming to, or suppression by, the fetal cells when the responses of lymphocytes from the mother and her HLA identical twin were compared. The possibility that these cells can protect the fetus from infection and/or stimulate the production of maternal anti-fetal HLA-antibodies is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankhurst A. D., Hastain E., Goodwin J. S., Peake G. T. The nature of the prostaglandin-producing mononuclear cell in human peripheral blood. J Lab Clin Med. 1981 Feb;97(2):179–186. [PubMed] [Google Scholar]
- Bienenstock A. N., Belsito D. V., Baer R. L., Rosen J., Possick L. E., Thorbecke G. J. Syngeneic mixed lymphocyte reactions to murine Fc receptor-bearing, nonadherent low density lymph node cells and epidermal Langerhans cells. Eur J Immunol. 1985 Apr;15(4):381–386. doi: 10.1002/eji.1830150414. [DOI] [PubMed] [Google Scholar]
- Brown P. J., Johnson P. M. Fc gamma-receptor activity of isolated human placental syncytiotrophoblast plasma membrane. Immunology. 1981 Feb;42(2):313–319. [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Johnson P. M. Macrophage populations in the human placenta and amniochorion. Clin Exp Immunol. 1984 Aug;57(2):393–403. [PMC free article] [PubMed] [Google Scholar]
- Edwards J. A., Jones D. B., Evans P. R., Smith J. L. Differential expression of HLA class II antigens on human fetal and adult lymphocytes and macrophages. Immunology. 1985 Jul;55(3):489–500. [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Austyn J., Stahl P. D., Gordon S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981 Jul 1;154(1):60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsum U., Klareskog L., Peterson P. A. Distribution of Ia-antigen-like molecules on non-lymphoid tissues. Scand J Immunol. 1979;9(4):343–349. doi: 10.1111/j.1365-3083.1979.tb03172.x. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. S., King C. R., Jr, Wood G. W. Evaluation of human chorionic trophoblast cells and placental macrophages as stimulators of maternal lymphocyte proliferation in vitro. J Reprod Immunol. 1984 Dec;6(6):377–391. doi: 10.1016/0165-0378(84)90047-0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., Bowers W. E. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982 Jul 1;156(1):1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb M. F., Lyons C. R., Nunez G., Ball E. J., Stastny P., Vial W., Lem V., Weissler J., Miller L. M. Human alveolar macrophages: HLA-DR-positive macrophages that are poor stimulators of a primary mixed leukocyte reaction. J Immunol. 1986 Jan;136(2):497–504. [PubMed] [Google Scholar]
- Loke Y. W., Eremin O., Ashby J., Day S. Characterization of the phagocytic cells isolated from the human placenta. J Reticuloendothel Soc. 1982 Apr;31(4):317–324. [PubMed] [Google Scholar]
- Meyer F. A., Laver-Rudich Z., Tanenbaum R. Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in Wharton's jelly. Biochim Biophys Acta. 1983 Feb 22;755(3):376–387. doi: 10.1016/0304-4165(83)90241-6. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Parker J. W. Oxidation-induced lymphocyte transformation. Cell. 1976 Jan;7(1):13–20. doi: 10.1016/0092-8674(76)90250-6. [DOI] [PubMed] [Google Scholar]
- Santiago-Schwarz F., Woodward J. G., Dixon J. F., Parker J. W., Frelinger J. A., O'Brien R. L. Low-density mononuclear cells. Potent stimulators of the human MLR. Transplantation. 1983 May;35(5):463–469. doi: 10.1097/00007890-198305000-00014. [DOI] [PubMed] [Google Scholar]
- Sargent I. L., Arenas J., Redman C. W. Maternal cell-mediated sensitisation to paternal HLA may occur, but is not a regular event in normal human pregnancy. J Reprod Immunol. 1987 Feb;10(2):111–120. doi: 10.1016/0165-0378(87)90070-2. [DOI] [PubMed] [Google Scholar]
- Spalding D. M., Koopman W. J., Eldridge J. H., McGhee J. R., Steinman R. M. Accessory cells in murine Peyer's patch. I. Identification and enrichment of a functional dendritic cell. J Exp Med. 1983 May 1;157(5):1646–1659. doi: 10.1084/jem.157.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton L., Gadd M., Mason D. Y., Redman C. W. Cells bearing class II MHC antigens in the human placenta and amniochorion. Immunology. 1986 May;58(1):23–29. [PMC free article] [PubMed] [Google Scholar]
- Sutton L., Mason D. Y., Redman C. W. HLA-DR positive cells in the human placenta. Immunology. 1983 May;49(1):103–112. [PMC free article] [PubMed] [Google Scholar]
- Uren S., Boyle W. Isolation of macrophages from human placenta. J Immunol Methods. 1985 Apr 8;78(1):25–34. doi: 10.1016/0022-1759(85)90326-6. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann T. G., Singh B., Carlson G. A. Allogeneic placenta is a paternal strain antigen immunoabsorbent. J Immunol. 1979 Jan;122(1):270–274. [PubMed] [Google Scholar]


