Abstract
Background
Morbidity management is a core component of the global programme for the elimination of lymphatic filariasis. In a double-blind clinical trial, the tolerability and efficacy of Daflon (500 mg) + DEC (25 mg) or DEC (25 mg) alone, twice daily for 90 days, was studied in 26 patients with bancroftian filarial lymphoedema.
Results
None of the patients in either drug group reported any adverse reaction throughout the treatment period (90 days). Haematological and biochemical parameters were within normal limits and there was no significant difference between the pre-treatment (day 0) and post-treatment (day 90) values. The group receiving Daflon showed significant reduction in oedema volume from day 90 (140.6 ± 18.8 ml) to day 360 (71.8 ± 20.7 ml) compared to the pre-treatment (day 0, 198.4 ± 16.5 ml) value. This accounted for a 63.8% reduction in oedema volume by day 360 (considering the pre-treatment (day 0) as 100%). In the DEC group, the changes in oedema volume (between day 1 and day 360) were not significant when compared to the pre-treatment (day 0) value. The percentage reduction at day 360 was only 9%, which was not significant (P > 0.05).
Conclusion
This study has shown that Daflon (500 mg, twice a day for 90 days) is both safe and efficacious in reducing oedema volume in bancroftian filarial lymphoedema. Further clinical trials are essential for strengthening the evidence base on the role of this drug in the morbidity management of lymphatic filariasis.
Background
Lymphatic filariasis (LF) is endemic in as many as 80 countries [1,2]. An estimated 1.1 billion people are at risk of infection, and there are approximately 120 million people with patent infection or disease round the globe [3-5]. In India alone, 553 million people are estimated to live in areas endemic for lymphatic filariasis and there are approximately 21 million people with symptomatic filariasis [6]. Progressive lymphoedema (from the early reversible stages to irreversible and complicated stages) associated with the increase in episodic attacks of acute adenolymphangitis (ADL) [7] is the most important cause of physical suffering, permanent disability and economic loss [8-10]. It has been estimated that there are some 16.02 million cases of lymphoedema caused by LF globally, and of these, 7.44 million (46.4%) live in India [3].
LF is recognized as one of six potentially eradicable diseases [11] and in 1997, the World Health Assembly (WHA) passed a resolution calling for it's global elimination as a public health problem [4,12].
The current strategy for the global elimination of LF recommended by the Global Alliance for the Elimination of Lymphatic Filariasis (GAELF) [1] has two major components: transmission control and morbidity management [13].
Transmission control
The most important transmission control strategy being implemented is the annual mass administration of single-dose anti-parasitic drugs (albendazole with diethylcarbamazine citrate (DEC) in countries where onchocerciasis or loiasis is not co-endemic with LF, or albendazole with ivermectin in countries where onchocerciasis or loaiasis is co-endemic with LF) to the entire at risk community, aimed at a significantly reducing community parasite load [14,15].
Morbidity management
For morbidity management, the current emphasis is on use of an appropriate hygiene and skin care regimen, (pioneered by Gerusa Dryer in Brasil), that lymphoedema patients can use everyday [16] for the prevention of episodic attacks of adenolymphangitis (ADL) and progression of disease [2,17].
Availability of other measures for morbidity management (such as drugs or physiotherapy or surgery) which can reduce oedema volume (along with appropriate hygiene and skin care) will be important in the alleviation of suffering and consequent improvement in psycho-social condition of these patients.
Daflon, (micronized purified flavonoid fraction of Rutaceae aurantiae) [18] has been used in clinical practice to treat a variety of lymphoedemas, such as post radical mastectomy oedema [19], chronic venous insufficiency [20], haemorrhoids [21,22], varicose ulcers [23,24], post-phlebitic syndrome, dysfunctional uterine bleeding [25] and idiopathic cyclic oedema syndrome [26].
To date, no clinical trials have been conducted to assess the suitability of this drug in the treatment of filarial lymphoedema. In this paper, we are presenting our observations on the tolerability and efficacy of Daflon (500 mg, twice a day for 90 days) in reducing oedema volume in patients with bancroftian filarial lymphoedema.
Materials and Methods
Selection of patients
Initially, patients with unilateral lower limb lymphoedema were detected by conducting a morbidity survey in a village known to be endemic for lymphatic filariasis near Pondicherry in south India (recording Wuchereria bancrofti microfilaria rate of 17.2%, an overall disease rate of 14.1%, lymphoedema rate of 6.3% and antigenaemia rate of 28.6% using ICT card test; Vector Control Research Centre – unpublished data). These patients were referred to the Government General Hospital at Pondicherry, where they were examined by a senior physician and recruited to the study as per inclusion/exclusion criteria (Table 1).
Table 1.
Inclusion/exclusion criteria used for recruitment of patients in the study.
| Inclusion criteria | Exclusion criteria |
| a) Patients with unilateral lower limb lymphoedema (of either grade I/II minimum for six months†) from area highly endemic for bancroftian filariasis. | a) Haemoglobin less than 10 gm/dl or haematocrit < 30%. |
| b) Patients looking apparently healthy except as (a) above and with normal body weight as per age. | b) Clinical exclusion of patients with lymphoedema due to any other known aetiology other than filariasis (such as venous insufficiency, varicose ulcers). |
| c) Patients with normal electro-cardiogram (ECG). | |
| d) Patients with normal chest X-ray. | c) Patients presenting with acute adenolymphangitis at the time of recruitment. |
| e) Results within normal limits in routine stool and urine examination. | d) Patients with a history of taking Daflon or DEC in the past 3 months. |
| f) Results within normal limits for haemoglobin concentration, total white blood cell count, differential count, absolute eosinophil count, erythrocyte sedimentation rate, packed cell volume. | e) Symptoms / signs of any other chronic illness |
| g) Results within normal limits for blood urea, sugar, bilirubin, creatinine, cholesterol, serum sodium, potassium, chloride, protein, albumin, globulin, pyruvic transaminase, alkaline phosphatase. | f) Patients with a history of any drug intolerance, reaction or allergy. |
†As per diagnostic criteria of WHO, 1992 (Ref. No. [31]). Grade I: oedema reversible on elevation. Grade II: oedema not reversible on elevation, skin not thickened.
In the natural history of LF, many patients with lymphoedema do not have demonstrable microfilaraemia [27-29] nor antigenaemia [30]. Therefore, in areas which are considered to be highly endemic for LF, (such as in the current study), cases of unilateral lymphoedema are considered to be of filarial origin by exclusion of all other conditions, (such as venous insufficiency and varicose ulcers, which could also present with lymphoedema), by careful history taking and clinical examination, using the clinical criteria for diagnosis and grading recommended by the World Health Organization (WHO) [31].
Study design and treatment regimen
Twenty-six patients (18 female and 8 male patients) between the ages of 20 and 55 years, (mean 39 years), who met the inclusion / exclusion criteria [Table 1], and who had given written informedconsent, were admitted to the Government General Hospital at Pondicherry for a period of four days and randomly allocated into one of two drug groups:
Group A: Daflon (500 mg) + DEC (25 mg) twice a day for 90 days
Group B: DEC (25 mg) twice a day for 90 days.
The drugs were repackaged in look-alike capsules containing either Daflon (500 mg) + DEC (25 mg) or DEC (25 mg).
The appropriate drug regimen was administered to the patients twice daily (morning and evening) from day 1 to day 3 (three days excluding day 0, i.e. day of admission) of hospitalization, under the direct supervision of the medical team. Patients were discharged on the morning of day 4 with a pack of the appropriate drug capsules (for the next 12 days: to complete the treatment up to day 15). The patients were educated and instructed by the physician and a social worker to comply with the dosage schedule and were asked initially to report every fortnight for measurement of oedema volume and to receive a further supply of drug capsules for the proceeding 15 days, up to day 90. Thereafter, patients were requested to attend 3 subsequent times, day(s) 180, 270 and 360 to allow measurement of oedema volume.
Ethical considerations
The study conformed to the principles of Helsinki Declaration II [32], the Guidelines for Good Clinical Practice (GCP) for Trials on Pharmaceutical Products [33] and the guidelines of the Indian Council of Medical Research for bio-medical research involving human subjects [34]. Furthermore, the study was approved by the Institutional Scientific Advisory Committee and the Institutional Ethical Committee. The study was "blind" to the extent that patients, clinicians evaluating the adverse effects, and laboratory staff carrying out the laboratory tests were unaware of the individual treatment schedules. Blinding and coding of the drugs was done by an independent monitor (a senior scientist who was not an investigator) after repacking in look-alike capsules by a pharmaceutical company in Pondicherry. The codes were broken only after completion of the study.
Assessment of results
Tolerability
All patients were clinically monitored for any adverse reactions (such as abdominal pain, nausea, vomiting, chest pain, arthralgia, diarrhoea, fever, headache, myalgia and chills) at 8 hourly intervals for first 24 hours and thereafter every 24 hours for further two days (until the end of day 3). All systemic reactions, if any, were recorded in a pre-designed form. Laboratory investigations on haematology and biochemistry parameters (haemoglobin concentration, total white blood count, differential count, absolute eosinophil count, erythrocyte sedimentation rate, packed cell volume, blood urea, sugar, bilirubin, creatinine, cholesterol, serum sodium, potassium, chloride, protein, albumin, globulin, glutamic pyruvic transaminase, alkaline phosphatase) were assessed on day 0, (pre-treatment), and on completion of treatment (day 90).
Efficacy
Oedema volume was recorded using a water displacement method [35] on day 0 (pre-treatment) and every fortnight from day 15 to day 90, and thereafter every 3 months on day(s) 180, 270 and 360.
On day 0, during the clinical history taking, the senior physician enquired about patient experiences of ADL attacks, and their frequency in the past 6 months prior to admission. At each follow-up point the patients were asked about the occurrence of ADL attacks in the period between the visits, and were also clinically examined for signs and symptoms of acute disease, as per WHO criteria [31].
Statistical analysis
The mean age of the patients and the mean frequency of ADL attacks (6 months prior to treatment) in the two drug groups were compared using independent t test. The statistical significance in the difference between the mean oedema volume was calculated using paired t-test.
Results
A total of 26 ambulatory patients with unilateral lymphoedema, (selected as per inclusion and exclusion criteria [Table 1]), were recruited to the study; 13 in each drug group (Group A: Daflon (500 mg) + DEC (25 mg) twice a day, for 90 days; Group B: DEC (25 mg) twice a day, for 90 days). The mean age (± SD) of the patients in the Daflon + DEC group was 40 (± 11.5) years (range 20–55) and in the DEC group it was 38 (± 6.8) years (range 24–50) (P > 0.05 between the two groups). There were 12 patients with grade II oedema and one patient with grade I oedema in each drug group. There was no significant difference in the pre-treatment (day 0) mean oedema volume (± SEM) between the Daflon + DEC (198.4 ± 16.5 ml) and the DEC alone (272.9 ± 48.0 ml) groups (P > 0.05). All the 26 patients completed the full 90 day treatment schedule.
Tolerability
None of the patients in either study group complained of any adverse reaction during the 90 days of treatment. The haematological and biochemical parameters were within normal limits for all patients, and did not vary significantly between pre-treatment (day 0) and on completion of treatment (day 90) (data not shown).
Efficacy
The mean oedema volume (± SEM) in the Daflon + DEC group was 140.6 (± 18.8) ml at the end of treatment period (day 90) and it was 71.8 (± 20.7) ml at the end of the follow-up period (day 360) (Figure 1). There was a significant difference in the mean oedema volume on day 90 and on day 360 in comparison to the pre-treatment value (198.4 ± 16.5 ml on day 0, P < 0.05) (Table 2). The mean oedema (± SEM) volume in the DEC group was 272.9 ± 48.0 ml the end of the follow-up period (day 360) (Figure 1). There was no significant difference in the mean oedema volumes on day 90 and on day 360 in comparison to the pre-treatment value (272.9 ± 48.0 ml on day 0, P > 0.05).
Figure 1.
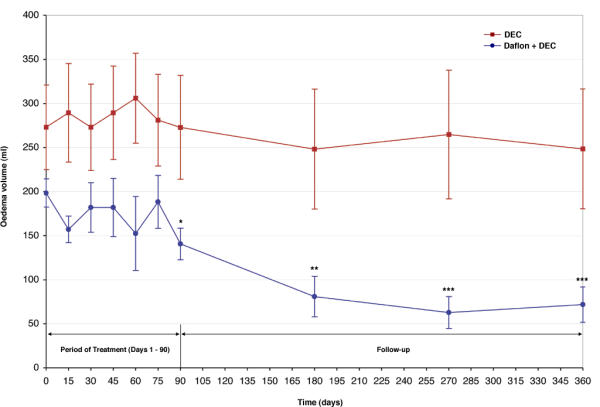
Comparison of changes in mean (± SEM) oedema volume (in ml) over 360 days between patients treated with Daflon (500 mg) + DEC (25 mg), and patients treated with DEC (25 mg), twice daily for 90 days. Day 0: pre-treatment Day 1 – day 90 period of treatment Day 91 – day 360 period of follow-up *P = < 0.05 vs. day 0 **P = < 0.005 vs. day 0 ***P = < 0.001 vs. day 0
Table 2.
Comparison of the day specific mean oedema volume between patients treated with Daflon (500 mg) + DEC (25 mg) and patients treated with DEC (25 mg), twice daily for 90 days.
| Days of comparison | Daflon 500 mg + DEC | DEC | ||
| t – value | P value | t – value | P value | |
| day 0 vs. day 75 | 0.316 | 0.758 | -0.140 | 0.891 |
| day 0 vs. day 90 | 2.928 | 0.013 | 0.001 | 0.999 |
| day 0 vs. day 180 | 4.149 | 0.002 | 0.521 | 0.612 |
| day 0 vs. day 270 | 4.989 | < 0.001 | 0.156 | 0.879 |
| day 0 vs. day 360 | 6.959 | < 0.001 | 0.428 | 0.676 |
| day 75 vs. day 90 | 2.574 | 0.026 | 0.433 | 0.673 |
| day 75 vs. day 180 | 3.309 | 0.007 | 1.291 | 0.221 |
| day 180 vs. day 270 | 0.621 | 0.547 | -1.003 | 0.336 |
| day 270 vs. day 360 | 0.325 | 0.751 | -0.006 | 0.996 |
Day 0: pre-treatment Day 1 – day 90: period of treatment Day 91 – day 360: period of follow-up
The percentage change of oedema volume in comparison to pre-treatment (day 0) (considering day 0 volume as 100%) in the two treatment groups is shown in Figure 2. It was observed that in the Daflon + DEC group the oedema volume reduced by 29.1% at the end of treatment period (day 90) and by 63.8% at the end of the follow-up period (day 360). On the other hand, in the DEC group the percentage reduction was nil at the end of the treatment period (day 90) and it was 9% at the end of the follow-up period (day 360).
Figure 2.
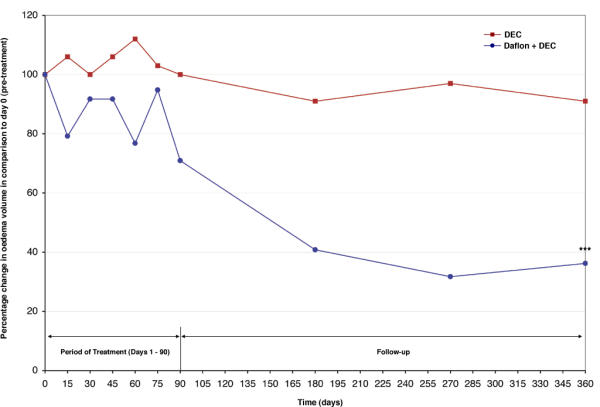
Comparison of percentage change in oedema volume (in ml) over 360 days between patients treated with Daflon (500 mg) + DEC (25 mg), and patients treated with DEC (25 mg), twice daily for 90 days. Day 0: pre-treatment Day 1 – day 90: period of treatment Day 91 – day 360: period of follow-up Day 0 oedema volume is considered as 100% *** P = < 0.001 vs. day 0
Comparison of the patterns of change in the day-specific mean oedema volume (Figure 1) and its percentage change (considering day 0 as 100%) (Figure 2) between the two treatment groups (Table 2) showed that in the Daflon + DEC group, maximum reduction was observed between day 75 to day 180 (significant reduction in oedema volume; t = 3.31, P < 0.01) and stabilized thereafter (no significant difference in oedema volume between day 180 versus day 270 or between day 180 versus day 360, P > 0.05). In the DEC group, the mean oedema volume was more or less stable through out the observation period (Table 2).
In the 6 month period prior to starting the treatment, the mean frequency of episodic ADL attacks (± SD) was 0.9 ± 1.1 in the Daflon + DEC group and it was 0.6 ± 0.9 in the DEC group (P > 0.05 between the two groups). None of the patients in either of the drug groups suffered from an ADL attack through out the treatment and follow-up period (i.e. between day 1 and day 360).
Discussion
The World Health Organisation has targeted lymphatic filariasis for elimination as a public health problem by the year 2020 [1,2]. India, which has the highest burden of LF, has set a target for national elimination of LF by the year 2015 [36]. Although annual mass administration of single-dose anti-filarial drugs to entire endemic communities for the control of transmission of lymphatic filariasis is being implemented in many counties, including India, (under which approximately 50 million citizens are currently being covered annually) [2,15], morbidity management [13] aimed at the alleviation of suffering of the individual patients has, in many countires, only recently begun to be addressed.
There is no doubt that the introduction of an appropriate hygiene and skin care regimen that patients can practice in their own environment will be most important in providing long term gains in the management of their morbidity. However, realisation of this strategy in many communities (living in rural and urban settings) in different endemic countries with wide socio-economic diversity remains a major challenge [17].
While the above strategy is being implemented, it is important to develop other measures, which could reduce oedema volume in patients with filarial lymphoedema, and, which can be integrated alongside a hygiene and skin care regimen.
Although, surgical procedures have been developed for filarial lymphoedema cases [37,38], these can only be performed in a few specialized centers, where expertise is available. Furthermore, it is costly and it has been observed that sustaining the gains achieved by surgery depends on the ability to prevent subsequent ADL episodes (by following a hygiene and skin care regimen) [38].
Physiotherapeutic measures such as manual massage, pneumatic compression and interferential current therapy have been found to be useful in odemea volume reduction in other secondary lymphoedema cases [39,40,43], however, these have yet to be properly evaluated in the management of filarial lymphoedema. Interferential current therapy showed significant oedema reduction in brugian filarial lymphoedema cases [41]. Although, pneumatic compression also results in oedema volume reduction, the results are not sustained [42]. Manual massage could be most useful as a self-help measure [43], but objective data are yet to be generated in LF cases. Of the chemotherapeutic agents investigated previously, 5,6 benzo-alpha-pyrone was most promising. In a double-blind placebo controlled study on bancroftian filarial lymphoedma cases in south India, it was observed that 5,6 benzo-alpha-pyrone (given at the dosage of 200 mg twice daily) resulted in significant reduction in oedema volume (63% in grade II cases) at the end of a two year treatment period [44]. The efficacy of this drug has also been demonstrated in a study in China [45]. However, 5,6 benzo-aplha-pyrone cannot be currently recommended for use as the drug has been shown to be hepato-toxic [46].
Earlier studies have shown that DEC has limited role in the management of filarial lymphoedema. In areas endemic for burgian filariasis in Indonesia considerable improvement in lymphoedema, including reversal of elephantiasis, has been reported [47]. However, these observations were based on community studies and objective measurement of oedema volume was not carried out. A significant reduction in oedema volume was reported with repeated courses of DEC along with supportive measures (such as pneumatic compression, use of crepe bandage etc.) in a study, again on brugian filarial lymphoedema cases, from south India [48]. However, this was an open trial and it is not possible to differentiate the effect of DEC alone from that of the supportive measures. In bancroftian filarial lymphoedema cases, long term DEC therapy (6 mg /kg /day in two divided doses for 2 years) resulted in reduction in oedema volume ranging only between 3 to 7 % in different grades of oedema [44]. A double blind clinical trial with single dose DEC (6 mg / kg body weight) or ivermectin (400 mg / kg body weight) did not show any significant change in oedema volume in bancroftian filarial lymphoedema cases over a one-year follow-up period (Vector Control Research Centre – unpublished data). In the current study, DEC treatment alone at the dosage of 25 mg twice a day for 90 days did not result in significant change oedema volume in filarial lymphoedema cases. Freedman et al., using lymphoscintigraphy did not observe any improvement in lymphatic pathology after two courses of DEC (for 12 days each) in bancroftian filarial lymphoedema cases [49]. Furthermore, DEC did not reduce the incidence of episodic ADL attacks in individual lymphoedema cases [50,51] as well as in the community after mass drug administration [52]. These results suggest the limitations of DEC in the morbidity management in LF.
This is the first report of a clinical trial on the tolerability and efficacy of Daflon in filarial lymphoedema cases. Daflon is known to be phlebotonic, it reduces capillary permeability and has an anti-lipidaemic effect [19,26]. This drug is known to be safe and without any adverse reaction in the dosage of 2 tablets of 500 mg each per day, and it has been given up to one-year period [53,54]. The results of the current study showed that there was neither any adverse reaction during the 90 days treatment period nor any significant change between the pre-treatment (day 0) and post-treatment (day 90) haematological or biochemical parameters in any of the patients (which were within normal limits). This demonstrates that Daflon (500 mg, twice a day for 90 days) is safe, well tolerated, and can be used up to 90 days in patients with filarial lymphoedema. Furthermore, the drug is also efficacious, reflected by a significant reduction in oedema volume of 63.8% (recorded between day 0 and day 360, inclusive of 90 days of treatment period) (Figure 2). A significant reduction in oedema volume was detected by the end of day 90 (Table 2). In other clinical conditions, appreciable change has been detected between 6 weeks to 6 months [55]. The pattern of change in oedema volume showed a marked reduction from day 75 until day 180 (Figure 1), after which the change was marginal (Table 2). This could suggest that repeat course(s) may be required for further decrease in oedema volume. Although Daflon was given along with DEC in the current study, since DEC alone did not show any significant reduction in oedema volume (Table 2, Figure 1), the results seen in the former group is likely to be due to Daflon.
None of the patients in either drugs group suffered from an ADL attack during the treatment and follow-up period. The reason for this, however, is not clear. Although DEC, (25 mg twice a day for 90 days), was given to all patients, it is difficult to assign this effect to DEC, as previous experience, in both hospital and community studies, did not favour this [50-52]. All the patients were also from a limited geographical area with similar environmental and socio-economic conditions having similar risk for ADL. None of the patients were advocated a specific hygiene and skin care regimen as a part of the protocol (since this is not yet a routine measure advocated by the National Filariasis Control Programme [56] and also as this could confound the results of effects of the drug (however, for ethical reasons all patients were educated in an appropriate hygiene and skin care regimen after completion of the study)).
We conclude that Daflon (500 mg, twice a day for 90 days) is both safe and efficacious in reducing oedema volume in filarial lymphoedema. DEC, at the dosage given, did not result in any significant change in oedema volume. However, as the current study was limited to a few patients in south India (and did not have an arm of Daflon alone) further clinical trials (preferably multi-centre) with larger numbers of patients need to be carried out (and should address the effect of Daflon on lymphatic pathology). This is essential for strengthening the evidence base on the use of Daflon in the management of filarial lymphoedema, before recommending its use in morbidity management to the Global Programme for the Elimination of Lymphatic Filariasis.
Competing interests
None declared
Authors' contributions
LKD; Study design, case detection, recruitment and follow-up, data analysis and manuscript preparation. GSR; Study of tolerability and efficacy in the hospital. SPP; Study concept, design, implementation and manuscript preparation.
Acknowledgments
Acknowledgements
The authors would like to thank Dr. Vijayan for his assistance in organizing the laboratory investigations and Dr. P. Vanamail, Senior Research Scientist, Vector Control Research Centre (VCRC) for his help in the statistical analysis and Dr P.K.Das, Director, VCRC for his initiative and administrative support. The authors acknowledge the gift of the Daflon by M/S Serdia Phramaceutical Ltd., Mumbai, India (coordinated by Dr. Prashant Desai).
The authors would also like to thank Mr. Palaniswamy of Caplin Laboratories Ltd., Pondicherry, India, for repacking the drugs used in the study.
The study was funded by the Indian Council of Medical Research, New Delhi, India http://icmr.nic.in/.
Contributor Information
LK Das, Email: lk_das@yahoo.com.
G Subramanyam Reddy, Email: msgh@pondy.pon.nic.in.
SP Pani, Email: sp_pani@vsnl.com.
References
- Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Health. 2000;5:591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- Das PK, Pani SP, Krishnamoorthy K. Prospects of elimination of lymphatic filariasis in India. ICMR Bulletin. 2002;32:41–54. [Google Scholar]
- Michael E, Bundy DAP, Grenfell BT. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112:409–428. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- Das PK, Pani SP. Towards elimination of lymphatic filariasis in India: Problems, challenges, opportunities and new initiatives. J Int Med Sci Acad. 2000;13:18–26. [Google Scholar]
- Ottesen EA, Duke BOL, Karam M, Behbehani K. Strategies and tools for the control /elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- Sabesan S, Palaniyandi M, Das PK, Michael E. Mapping of lymphatic filariasis in India. Ann Trop Med Parasitol. 2000;94:591–606. doi: 10.1080/00034983.2000.11813582. [DOI] [PubMed] [Google Scholar]
- Pani SP, Yuvraj J, Vanamail P, Dhanda V, Michael E, Grenfell BT, Bundy DAP. Episodic adenolymphangitis and lymphoedema in patients with bancroftian filariasis. Trans R Soc Trop Med Hyg. 1995;89:72–74. doi: 10.1016/0035-9203(95)90666-5. [DOI] [PubMed] [Google Scholar]
- Ramaiah KD, Vijay Kumar KN, Ramu K, Pani SP, Das PK. Functional impairment caused by lymphatic filariasis in rural areas of south India. Trop Med Int Health. 1997;2:832–838. doi: 10.1046/j.1365-3156.1997.d01-406.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization The state of world health. World Health Report-Bridging the gaps (WHO, Geneva) 1995;1:42. [Google Scholar]
- Ramaiah KD, Das PK, Michael E, Guyatt H. The Economic Burden of Lymphatic Filariasis in India. Parasitology Today. 2000;16:251–253. doi: 10.1016/S0169-4758(00)01643-4. [DOI] [PubMed] [Google Scholar]
- Centre for Disease Control (CDC) Recommendations of the International Task Force for Disease Eradication. Morbidity and Mortality Weekly Report. 1993;42:1–38. [PubMed] [Google Scholar]
- World Health Organization Resolution of the Executive Board of the WHO: Elimination of Lymphatic Filariasis as a Public Health Problem. Fiftieth World Health Assembly, Geneva. 13 May 1997.
- Dreyer G, Addiss D, Bettinger J, Dreyer P, Noroes J, Rio F. Introduction. Lymphoedema Staff Manual: Treatment and Prevention of problems Associated with Lymphatic Filariasis (WHO, Geneva) 2000. pp. v–vii.
- Ottesen EA, Ismail MM, Horton J. The role of Albendazole in programmes to eliminate lymphatic filariasis. Parasitology Today. 1999;15:382–386. doi: 10.1016/S0169-4758(99)01486-6. [DOI] [PubMed] [Google Scholar]
- Pani SP, Subramanyam Reddy G, Das LK, Vanamail P, Hoti SL, Ramesh J, Das PK. Tolerability and efficacy of single dose albendazole, diethylcarbamazine (DEC) or co-administration of albendazole with DEC in the clearance of Wuchereria bancrofti in asymptomatic microfilaraemic volunteers in Pondicherry, South India: a hospital-based study. Filaria Journal. 2002;1 doi: 10.1186/1475-2883-1-1. http://www.Filariajournal.com/content/1/1/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of Lymphatic Disease in Bancroftian Filariasis: A Clinical Perspective. Parasitology Today. 2000;16:544–548. doi: 10.1016/S0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- Das PK, Pani SP. Towards elimination of lymphatic filariasis in India, problems, challenges, opportunities and new initiatives. J Int Med Sci Acad. 2000;13:18–26. [Google Scholar]
- BIO-GARD Drug acting on alimentary system. Current Index of Medical Specialities. 1998;21:56–95. [Google Scholar]
- Pecking AP, Fevier B, Wargon C, Pillion G. Efficacy of Daflon 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer). Angiology. 1997;48:93–98. doi: 10.1177/000331979704800115. [DOI] [PubMed] [Google Scholar]
- Le Deyehat C, Khodabandehlou T, Vimeux M, Kempf C. Evaluation of haemorrhagical and microcirculatory disturbances in chronic venous insufficiency: activity of Daflon 500 mg. Int J Microcirc Clin Exp. 1997;17:27–33. doi: 10.1159/000179264. [DOI] [PubMed] [Google Scholar]
- Cospite M. Double-blind placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology. 1994;45:566–573. [PubMed] [Google Scholar]
- Meyer OC. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology. 1994;45:579–584. doi: 10.1177/000331979404500614. [DOI] [PubMed] [Google Scholar]
- Colgan MP, Moore DJ, Shanik DG. New approaches in the medical management of venous ulceration. Angiology. 1993;44:138–142. doi: 10.1177/000331979304400208. [DOI] [PubMed] [Google Scholar]
- Guilhou JJ, Debure O, Marzin L, Ouvry P, Zuccarelli F, Debure C, Van Landuyt H, Gillet-Terver MN, Guillot B, Levesque H, Mignot J, Pillion G, Fevrier F, Dubeaux D. Efficacy of Daflon 500 mg in venous leg ulcer healing: a double-blind, randomized, controlled versus placebo trial in 107 patients. Angiology. 1997;48:77–85. doi: 10.1177/000331979704800113. [DOI] [PubMed] [Google Scholar]
- Struckmann JR. Clinical efficacy of micronized flavonoid fraction: an overview. J Vasc Res. 1999;36:37–41. doi: 10.1159/000054072. [DOI] [PubMed] [Google Scholar]
- Pecking AP. Evaluation by lymphoscintigraphy of the effect of a micronized flavonoid fraction (Daflon 500 mg) in the treatment of upper limb lymphedema. Int Angiol. 1995;14:39–43. [PubMed] [Google Scholar]
- Pani SP, Srividya A, Rajagopalan PK. Clinical manifestations of bancroftian filariasis in relation to microfilaraemic and Diethylcarbamazine therapy. The National Medical Journal of India. 1991;4:9–14. [PubMed] [Google Scholar]
- Pani SP, Dhanda V. Natural history and dynamics of progression of clinical manifestation of filariasis. In: Kumar S, Sen AK, Dutta GP, Sharma RN, editor. Tropical Disease: Molecular Biology and Control Strategies. Publication and Information Directorate, CSIR, New-Delhi; 1994. pp. 1–8. [Google Scholar]
- Pani SP, Lall R. Clinical features, pathogenesis and management of lymphatic filariasis. ICMR Bulletin. 1998;28:41–51. [Google Scholar]
- Hoti SL, Elango A, Radjame K, Yuvraj J, Pani SP. Utility of filter paper samples in the detection of day blood filarial antigens by Og4C3 ELISA test: sensitivity during different periods of the day. The National Medical Journal of India. 2002;15:197–201. [PubMed] [Google Scholar]
- World Health Organization Informal consultation on evaluation of morbidity in lymphatic filariasis. WHO/TDR/FIL/MAD/923. 1992. pp. 1–8.
- World Medical Association Declaration of Helsinki – Recommendations guiding physicians in biomedical research involving human subjects. WHO Technical Report Series, No. 850, Annex 3. 1995. pp. 30–33.http://www.who.int/medicines/library/par/ggcp/GCPGuidePharmatrials.pdf
- World Health Organization Guidelines for good clinical practice (GCP) for trials on pharmaceutical products. WHO Technical Report Series, No 850, Annex 3. 1995. pp. 1–35.http://www.who.int/medicines/library/par/ggcp/GCPGuidePharmatrials.pdf
- Indian Council of Medical Research Statement of general principles on ethical considerations involving human subjects. Ethical guidelines for biomedical research on human subjects. 2000. pp. 1–8.
- Pani SP, Vanamail P, Yuvraj J. Limb circumference measurement for recording edema volume in patients with filarial lymphedema. Lymphology. 1995;28:57–63. [PubMed] [Google Scholar]
- Ministry of Health and Family Welfare Government of India National Health Policy. 2002. http://mohfw.nic.in/np2002.htm
- Binoy C, Govardha Rao V, Ananthakrishnan N, Kate V, Yuvraj J, Pani SP. Omentoplasty in the management of filarial lymphoedema. Trans R Soc Trop Med Hyg. 1998;92:317–319. doi: 10.1016/s0035-9203(98)91028-8. [DOI] [PubMed] [Google Scholar]
- Govardhan Rao V, Ananthakrishnan N, Pani SP, Kate V, Yuvraj J, Krishnamoorthy K. Factors influencing response to lymphonodo-venous shunt in filarial lymphoedema. The National Medical Journal of India. 1999;12:55–58. [PubMed] [Google Scholar]
- Foldi E, Foldi M, Clodius L. The lymphoedema Chaos: A Lancet. Annals of Plastic Surgery. 1989;22:505–515. doi: 10.1097/00000637-198906000-00007. [DOI] [PubMed] [Google Scholar]
- Zelikovski A, Deutsch A, Reiss R. The sequential pneumatic compression device in surgery for lymphedema in the limbs. J Cardiovas Surg (Tarino) 1993;24:122–126. [PubMed] [Google Scholar]
- Vector Control Research Centre Brugian filariasis and its control in Cherthala, Kerala state. Annual Report. 1994. pp. 14–18.
- Manjula Y, Kate V, Ananthkrishnan N. Evaluation of sequential intermittent pneumatic compression in filarial lymphoedema. The National Medical Journal of India. 2002;15:192–194. [PubMed] [Google Scholar]
- Pani SP, Yuvraj J, Vijayalakshmi Medical Management of lymphatic filariasis. A manual for clinicians (Vector Control Research Centre, Misc publication) 1997;21:1–25. [Google Scholar]
- Casley-Smith JR, Jamal S, Casley-Smith R Judith. Reduction of filaritic lymphoedema and elephantiasis by 5, 6-benzo-alpha-pyrone (coumarin), and the effects of Diethylcarbamazine (DEC). Ann Trop Med & Parasitol. 1993;87:247–258. doi: 10.1080/00034983.1993.11812763. [DOI] [PubMed] [Google Scholar]
- Casley-Smith JR, Wang CT, Zi-hai Cui. Treatment of filarial lymphoedema and elephantiasis with 5, 6-benzo-alpha-pyrone (coumarin). BMJ. 1993;307:1037–1041. doi: 10.1136/bmj.307.6911.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization "Coumarin (Lodema).". WHO Pharmaceutical Newsletter. 1996;10:2. [Google Scholar]
- Partono F. Filariasis in Indonesia. Clinical manifestations and basic concepts of treatment and control. Trans R Soc Trop Med Hyg. 1984;75:9–12. doi: 10.1016/0035-9203(84)90161-5. [DOI] [PubMed] [Google Scholar]
- Pani SP, Krishnamoorthy K, Prathibha J, Rao AS. Diethylcarbamazine and supportive measures for the treatment of Brugian filariasis. The National Medical Journal of India. 1989;2:260–263. [Google Scholar]
- Freedman David D, Bui Thuy, De Almeida Fiho Paulo, Braga Cynthia, Mala e Silva Maria Carmelita, Maciel Amelia, Furtado Aadre E. Lymphoscintigraphic assessment of the effect of Diethylcarbamazine treatment on lymphatic damage in human bancroftian filariasis. Am J Trop Med Hyg. 1995;52:258–261. doi: 10.4269/ajtmh.1995.52.258. [DOI] [PubMed] [Google Scholar]
- Shenoy RK, Suma TK, Rajan K, Kumaraswami V. Prevention of acute adenolymphangitis in brugian filariasis: comparison of the efficacy of ivermectin and Diethylcarbamazine, each combine with local treatment of the affected limb. Ann Trop Med Parasitol. 1998;92:587–594. doi: 10.1080/00034989859285. [DOI] [PubMed] [Google Scholar]
- Shenoy RK, Kumaraswami V, Suma TK, Rajan K, Radhakuttyamma G. A double blind placebo-controlled study, of efficacy of oral penicillin, diethylcarbamazine, or local treatment of the affected limb in preventing acute adenolymphangitis in lymphoedema caused by brugian filariasis: Ann Trop Med Parasitol. 1999;93:367–377. doi: 10.1080/00034989958366. [DOI] [PubMed] [Google Scholar]
- Das PK, Ramaiah KD, Vanamail P, Pani SP, Yuvraj J, Balarajan K, Bundy DAP. Placebo-controlled community trial of four cycles of single dose Diethylcarbamazine or ivermectin against Wuchereria bancrofti infection and transmission in India. Trans R Soc Trop Med Hyg. 2001;95:336–341. doi: 10.1016/s0035-9203(01)90260-3. [DOI] [PubMed] [Google Scholar]
- Filis FA, Georgopoulis SE, Papas SC, Votteas V, Bastounis FA. Therapeutic efficacy of flavonoids in oedema following reperfusion on acutely ischaemic legs. Int Angiol. 1999;18:327–30. [PubMed] [Google Scholar]
- Godeberge P. Daflon 500 mg in the treatment of hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology. 1994;45:574–578. [PubMed] [Google Scholar]
- Olszewski WL. Treatment and complications of lymphoedema. Atlas of the lymphatics of the lower limbs. 2000. pp. 67–94.
- Directorate of National Malaria Eradication Programme National Filariasis Control Programme India. Operational Manual. 1995. pp. 1–127.


