Abstract
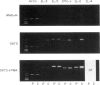
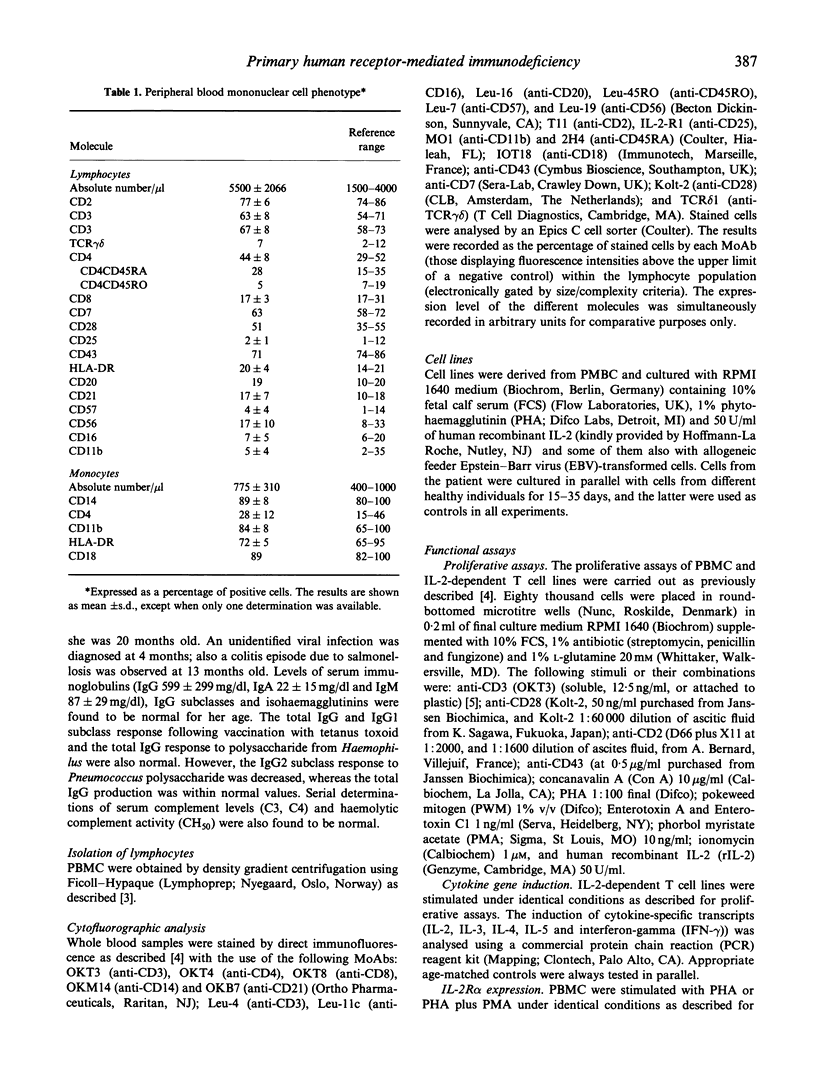
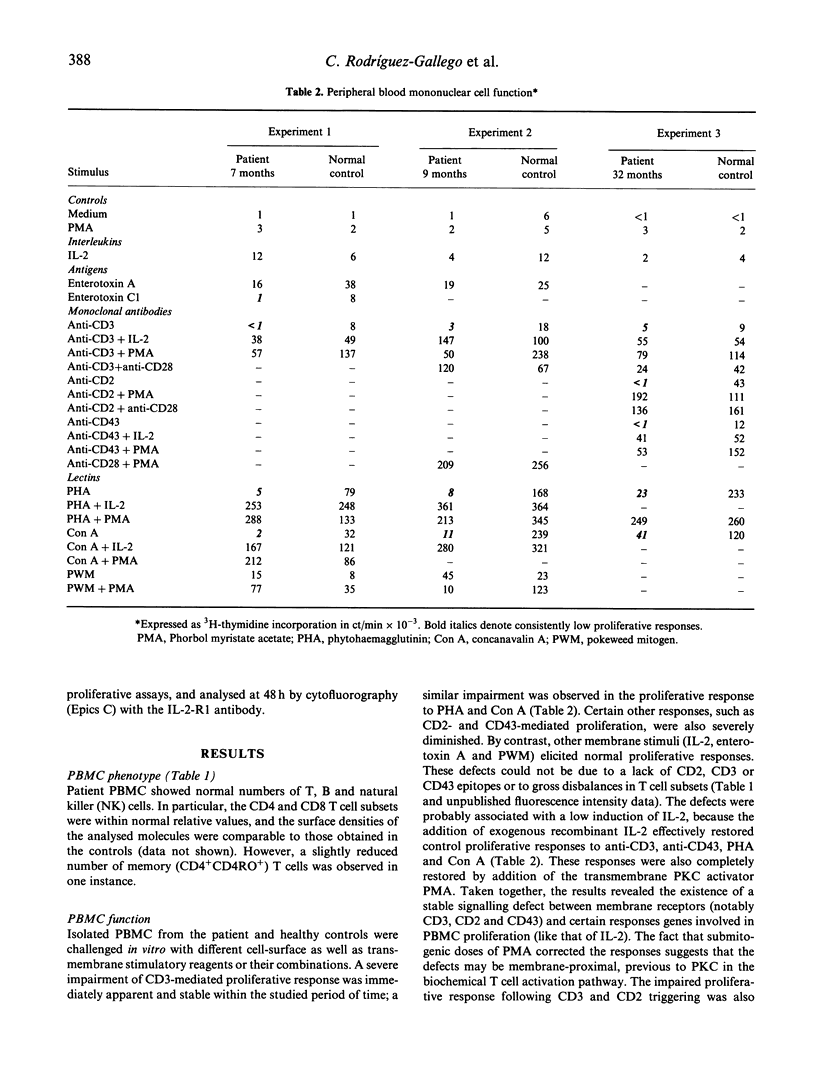
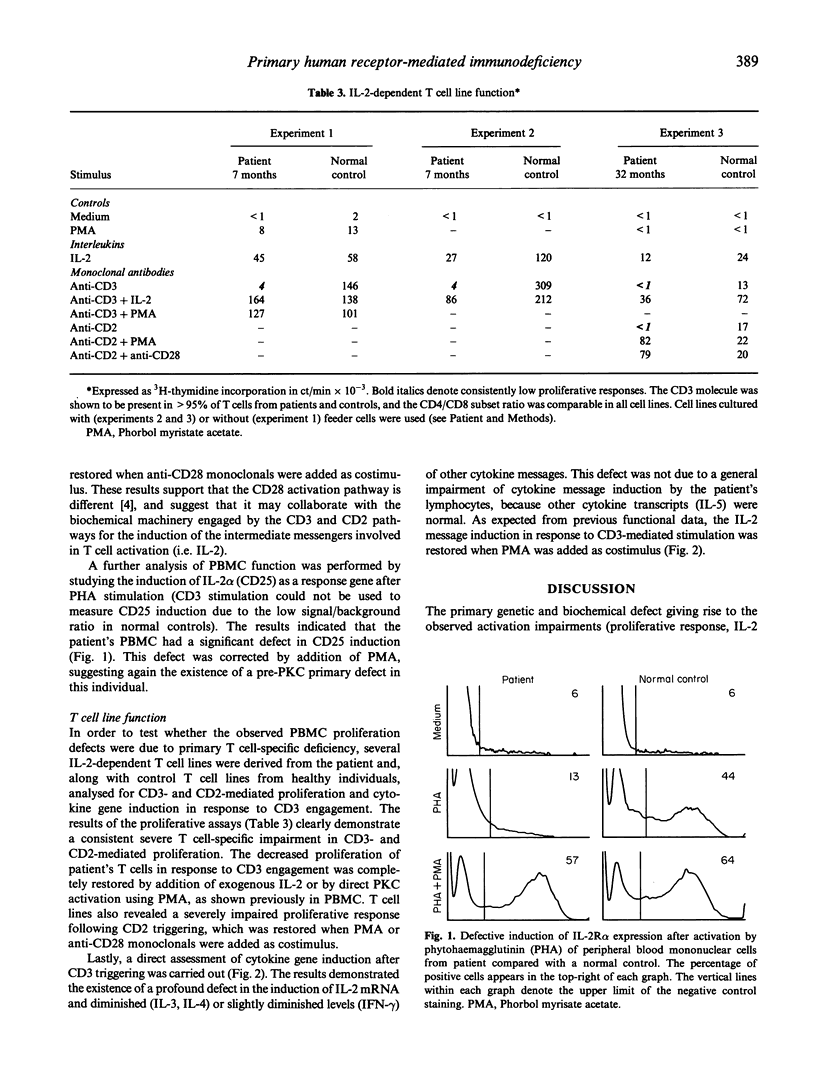
A 2-year-old female with important signs of immune response failure against virus, bacteria, fungi and protozoa and no obvious humoral or lymphocyte phenotypical defect was studied. Both peripheral blood mononuclear cells and IL-2-dependent T cell lines derived from the patient showed a severe selective T cell activation impairment via CD2, CD3 and CD43; however, this defect was reversible with the addition of either IL-2, or phorbol myristate acetate (PMA) or anti-CD28 antibodies. Concordantly, the induction of IL-2 (and, in part, IL-3 and IL-4) messenger RNA was severely reduced in stimulated T cells, but that of other cytokines was either normal (IL-5) or only slightly diminished (interferon-gamma (IFN-gamma)). It is concluded that an activation T cell defect exists previous to protein kinase C (PKC) and between membrane receptors and the activation pathway of certain response genes encoding for interleukins involved in proliferation (i.e. IL-2, IL-3 and IL-4), but not of others (i.e. IL-5). The use of T cell lines from human T lymphocyte activation deficiencies allows dissection of T cell pathology and the corresponding physiological pathways. In the present description, there is an evident independence of the CD28 T cell activation pathway from those induced through CD2 or CD3, and the differential gene regulation of the different interleukins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Coggeshall K. M., Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- Arnaiz-Villena A., Timón M., Rodríguez-Gallego C., Pérez-Blas M., Corell A., Martín-Villa J. M., Regueiro J. R. Human T-cell activation deficiencies. Immunol Today. 1992 Jul;13(7):259–265. doi: 10.1016/0167-5699(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Castigli E., Pahwa R., Good R. A., Geha R. S., Chatila T. A. Molecular basis of a multiple lymphokine deficiency in a patient with severe combined immunodeficiency. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4728–4732. doi: 10.1073/pnas.90.10.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila T., Castigli E., Pahwa R., Pahwa S., Chirmule N., Oyaizu N., Good R. A., Geha R. S. Primary combined immunodeficiency resulting from defective transcription of multiple T-cell lymphokine genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10033–10037. doi: 10.1073/pnas.87.24.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila T., Wong R., Young M., Miller R., Terhorst C., Geha R. S. An immunodeficiency characterized by defective signal transduction in T lymphocytes. N Engl J Med. 1989 Mar 16;320(11):696–702. doi: 10.1056/NEJM198903163201104. [DOI] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSanto J. P., Keever C. A., Small T. N., Nicols G. L., O'Reilly R. J., Flomenberg N. Absence of interleukin 2 production in a severe combined immunodeficiency disease syndrome with T cells. J Exp Med. 1990 May 1;171(5):1697–1704. doi: 10.1084/jem.171.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi S., Saiki O., Tanaka T., Ha-Kawa K., Igarashi T., Fujita T., Taniguchi T., Kishimoto S. Cellular and genetic analyses of IL-2 production and IL-2 receptor expression in a patient with familial T-cell-dominant immunodeficiency. Clin Immunol Immunopathol. 1988 Jan;46(1):24–36. doi: 10.1016/0090-1229(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Gascan H., Gauchat J. F., Aversa G., Van Vlasselaer P., de Vries J. E. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol. 1991 Jul 1;147(1):8–13. [PubMed] [Google Scholar]
- Granelli-Piperno A., Nolan P. Nuclear transcription factors that bind to elements of the IL-2 promoter. Induction requirements in primary human T cells. J Immunol. 1991 Oct 15;147(8):2734–2739. [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hengel H., Wagner H., Heeg K. Triggering of CD8+ cytotoxic T lymphocytes via CD3-epsilon differs from triggering via alpha/beta T cell receptor. CD3-epsilon-induced cytotoxicity occurs in the absence of protein kinase C and does not result in exocytosis of serine esterases. J Immunol. 1991 Aug 15;147(4):1115–1120. [PubMed] [Google Scholar]
- Kabelitz D., Pohl T., Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993 Jul;14(7):338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Beverly B., Tran A. C., Brorson K., Schwartz R. H., Lenardo M. J. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992 Aug 21;257(5073):1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Perandones C. E., Illera V. A., Peckham D., Stunz L. L., Ashman R. F. Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol. 1993 Oct 1;151(7):3521–3529. [PubMed] [Google Scholar]
- Pérez-Aciego P., Alarcón B., Arnaiz-Villena A., Terhorst C., Timón M., Segurado O. G., Regueiro J. R. Expression and function of a variant T cell receptor complex lacking CD3-gamma. J Exp Med. 1991 Aug 1;174(2):319–326. doi: 10.1084/jem.174.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Blas M., Arnaiz-Villena A., Góngora R., Segurado O. G., Vivanco J. L., Regueiro J. R. Impaired T cell signal transduction through CD28 in a patient with idiopathic thrombocytopenia. Clin Exp Immunol. 1991 Sep;85(3):424–428. doi: 10.1111/j.1365-2249.1991.tb05743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regueiro J. R., Perez-Aeiego P., Aparicio P., Martinez C., Morales P., Arnaiz-Villena A. Low IgG2 and polysaccharide response in a T cell receptor expression defect. Eur J Immunol. 1990 Nov;20(11):2411–2416. doi: 10.1002/eji.1830201108. [DOI] [PubMed] [Google Scholar]
- Regueiro J. R., Timón M., Pérez-Aciego P., Corell A., Martín-Vílla J. M., Rodríguez-Gallego C., Góngora R., Arnaiz-Villena A. From pathology to physiology of the human T-lymphocyte receptor. Scand J Immunol. 1992 Sep;36(3):363–369. doi: 10.1111/j.1365-3083.1992.tb02950.x. [DOI] [PubMed] [Google Scholar]
- Rijkers G. T., Scharenberg J. G., Van Dongen J. J., Neijens H. J., Zegers B. J. Abnormal signal transduction in a patient with severe combined immunodeficiency disease. Pediatr Res. 1991 Mar;29(3):306–309. doi: 10.1203/00006450-199103000-00017. [DOI] [PubMed] [Google Scholar]
- Rouleau M., Bernard A., Lantz O., Vernant J. P., Charpentier B., Senik A. Apoptosis of activated CD8+/CD57+ T cells is induced by some combinations of anti-CD2 mAb. J Immunol. 1993 Oct 1;151(7):3547–3556. [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992 Dec 24;71(7):1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Weinberg K., Parkman R. Severe combined immunodeficiency due to a specific defect in the production of interleukin-2. N Engl J Med. 1990 Jun 14;322(24):1718–1723. doi: 10.1056/NEJM199006143222406. [DOI] [PubMed] [Google Scholar]