Abstract
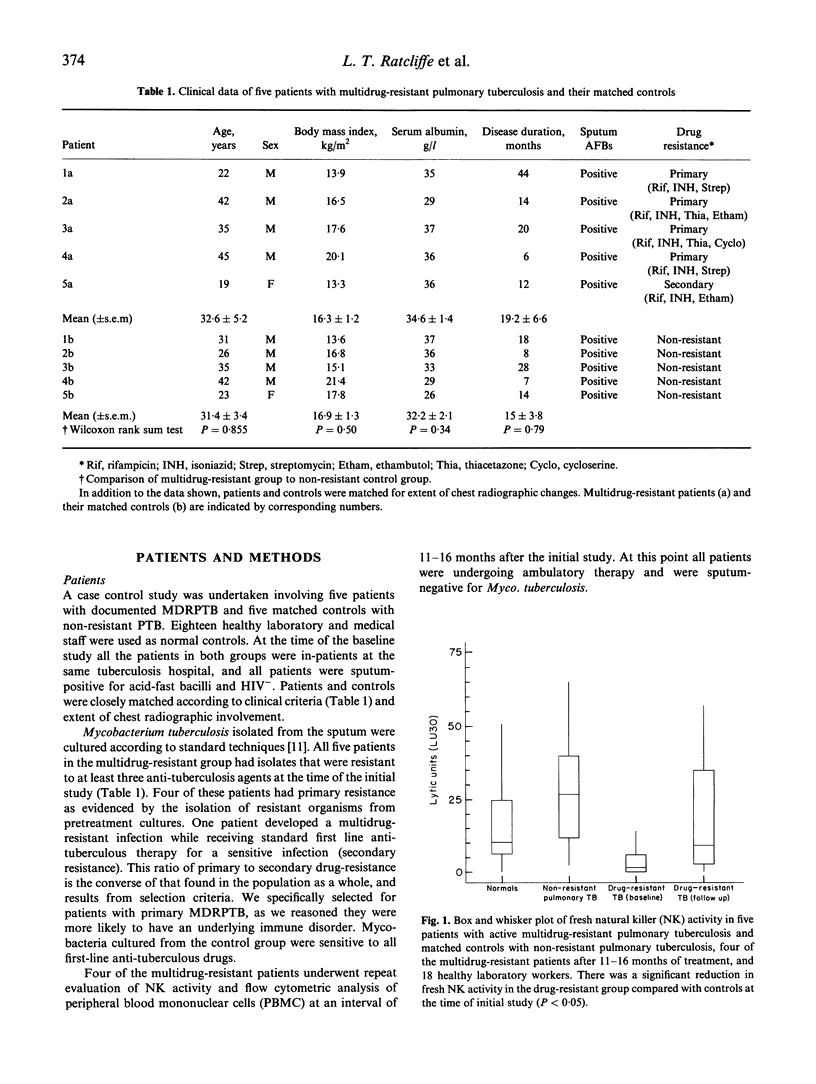
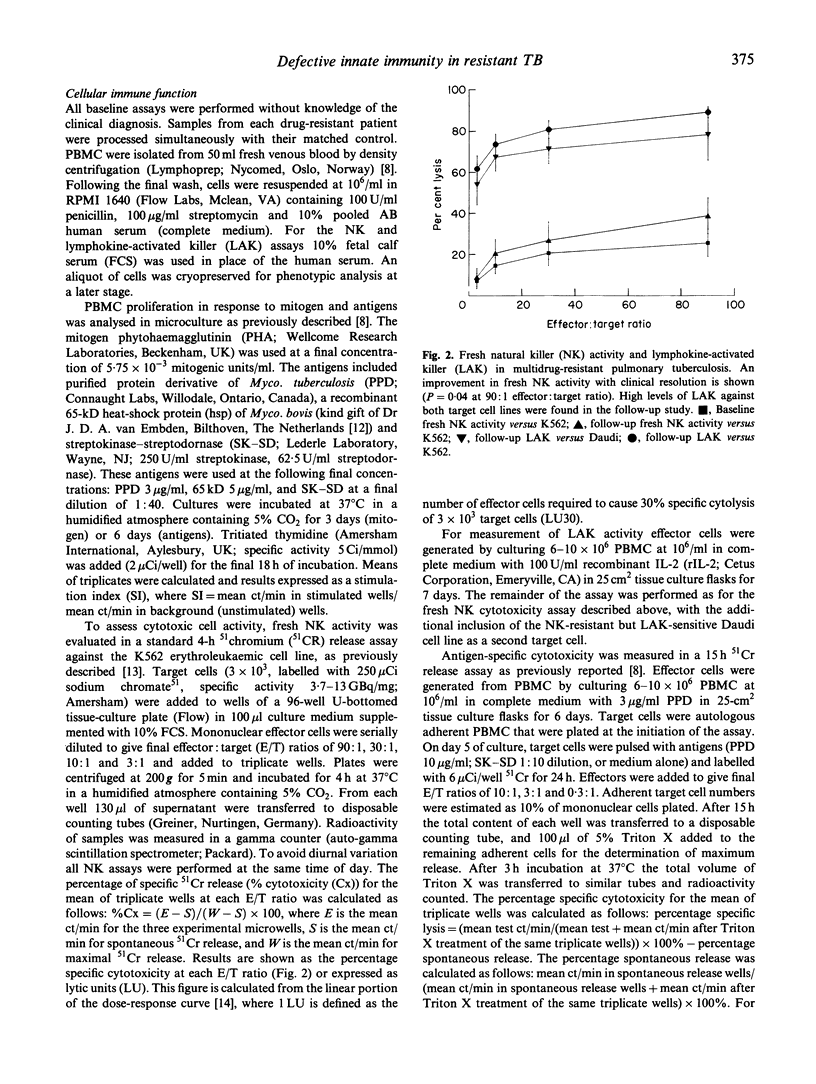
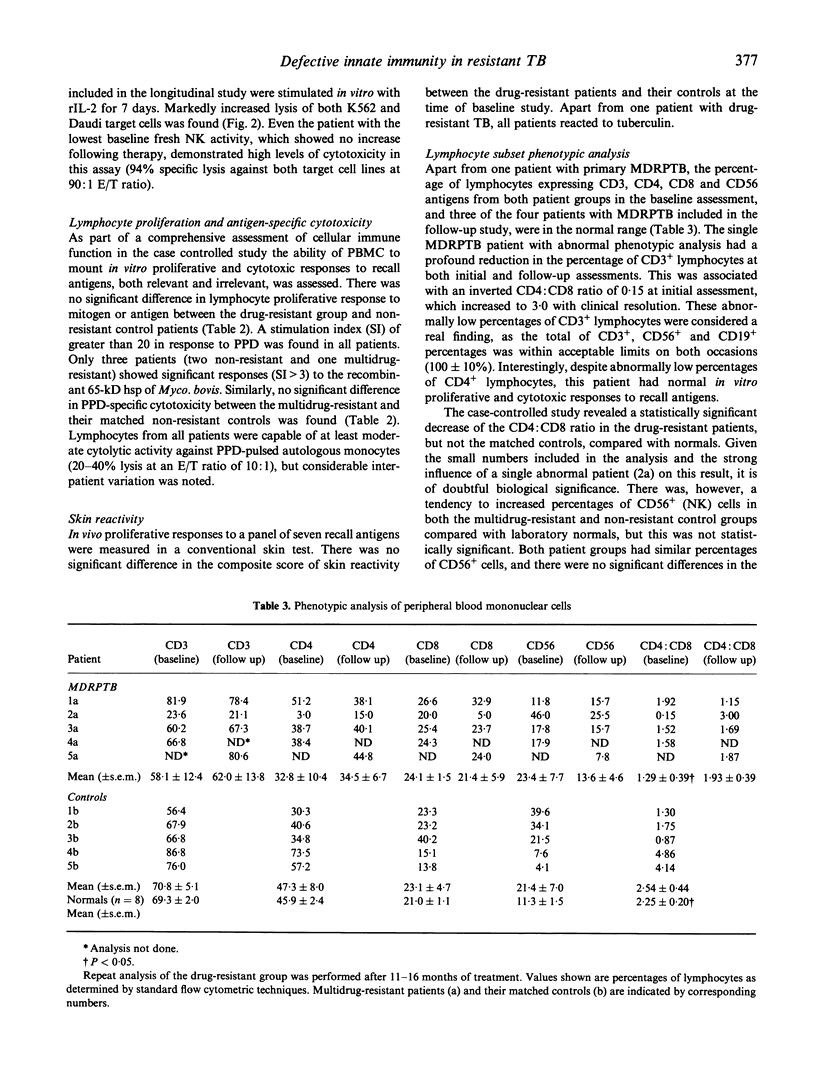
There has been a global increase in the incidence of multidrug-resistant pulmonary tuberculosis (TB). As there are no previous reports of immune function in HIV- patients with multidrug-resistant pulmonary TB, a comprehensive assessment of cellular immunity in this setting was undertaken. This involved a prospective, case-controlled study which included five patients with active multidrug-resistant pulmonary TB and five matched controls with active non-resistant infection, and documented the changes in immune parameters which occurred upon clinical resolution. Patients with multidrug-resistant TB had significantly lower fresh natural killer (NK) cell activity than matched controls with non-resistant pulmonary TB (P < 0.05). This was a specific abnormality, as there were no significant differences in antigen-specific cytotoxicity or lymphocyte proliferation in the case-controlled study. Follow-up assessment of the patients with multidrug-resistant infections indicated that clinical improvement correlated with a moderate increase in NK cell activity. Impaired NK cell function may be involved in the pathogenesis of multidrug-resistant TB.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E., Young L. S. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991 Jan 1;146(1):265–270. [PubMed] [Google Scholar]
- Bloom B. R. Tuberculosis. Back to a frightening future. Nature. 1992 Aug 13;358(6387):538–539. doi: 10.1038/358538b0. [DOI] [PubMed] [Google Scholar]
- Canetti G., Fox W., Khomenko A., Mahler H. T., Menon N. K., Mitchison D. A., Rist N., Smelev N. A. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41(1):21–43. [PMC free article] [PubMed] [Google Scholar]
- Chehimi J., Starr S. E., Frank I., Rengaraju M., Jackson S. J., Llanes C., Kobayashi M., Perussia B., Young D., Nickbarg E. Natural killer (NK) cell stimulatory factor increases the cytotoxic activity of NK cells from both healthy donors and human immunodeficiency virus-infected patients. J Exp Med. 1992 Mar 1;175(3):789–796. doi: 10.1084/jem.175.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S. W., Jarvis W. R., Martone W. J., Snider D. E., Jr Multidrug-resistant tuberculosis. Ann Intern Med. 1992 Aug 1;117(3):257–259. doi: 10.7326/0003-4819-117-3-257. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Uttamchandani R. B., Daikos G. L., Poblete R. B., Moreno J. N., Reyes R. R., Boota A. M., Thompson L. M., Cleary T. J., Lai S. An outbreak of tuberculosis caused by multiple-drug-resistant tubercle bacilli among patients with HIV infection. Ann Intern Med. 1992 Aug 1;117(3):177–183. doi: 10.7326/0003-4819-117-3-177. [DOI] [PubMed] [Google Scholar]
- Grange J. M. Drug resistance and tuberculosis elimination. Bull Int Union Tuberc Lung Dis. 1990 Jun-Sep;65(2-3):57–59. [PubMed] [Google Scholar]
- Harshan K. V., Gangadharam P. R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991 Aug;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kumararatne D. S., Pithie A. S., Drysdale P., Gaston J. S., Kiessling R., Iles P. B., Ellis C. J., Innes J., Wise R. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal T cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990 Jun;80(3):314–323. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorgat F., Keraan M. M., Lukey P. T., Ress S. R. Evidence for in vivo generation of cytotoxic T cells. PPD-stimulated lymphocytes from tuberculous pleural effusions demonstrate enhanced cytotoxicity with accelerated kinetics of induction. Am Rev Respir Dis. 1992 Feb;145(2 Pt 1):418–423. doi: 10.1164/ajrccm/145.2_Pt_1.418. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Savary C. A. Interleukin-2 corrects defective NK activity of patients with leukemia. Comp Immunol Microbiol Infect Dis. 1986;9(2-3):169–175. doi: 10.1016/0147-9571(86)90009-3. [DOI] [PubMed] [Google Scholar]
- Morikawa F., Nakano A., Nakano H., Oseko F., Morikawa S. Enhanced natural killer cell activity in patients with pulmonary tuberculosis. Jpn J Med. 1989 May-Jun;28(3):316–322. doi: 10.2169/internalmedicine1962.28.316. [DOI] [PubMed] [Google Scholar]
- Ota T., Okubo Y., Sekiguchi M. Analysis of immunologic mechanisms of high natural killer cell activity in tuberculous pleural effusions. Am Rev Respir Dis. 1990 Jul;142(1):29–33. doi: 10.1164/ajrccm/142.1.29. [DOI] [PubMed] [Google Scholar]
- Pearson M. L., Jereb J. A., Frieden T. R., Crawford J. T., Davis B. J., Dooley S. W., Jarvis W. R. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis. A risk to patients and health care workers. Ann Intern Med. 1992 Aug 1;117(3):191–196. doi: 10.7326/0003-4819-117-3-191. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Ross B. C., Dwyer B., Goh K. S., Clavel-Sérès S., Jeantils V., Cruaud P. Emergence during unsuccessful chemotherapy of multiple drug resistance in a strain of Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis. 1992 Oct;11(10):901–907. doi: 10.1007/BF01962370. [DOI] [PubMed] [Google Scholar]
- Ress S. R., Strassmann G., Bach F. H. HLA-DR expression on cytotoxic T lymphocytes. Scand J Immunol. 1985 Nov;22(5):455–461. doi: 10.1111/j.1365-3083.1985.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Snider D. E., Jr, Roper W. L. The new tuberculosis. N Engl J Med. 1992 Mar 5;326(10):703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


