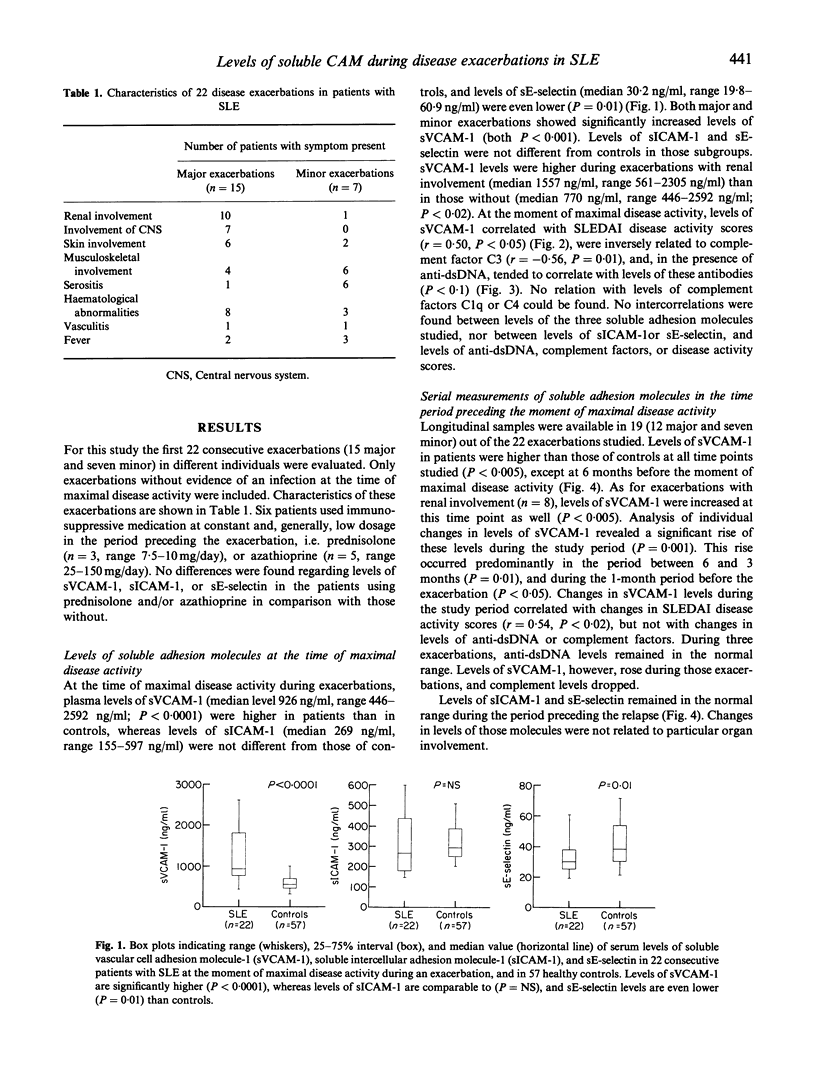
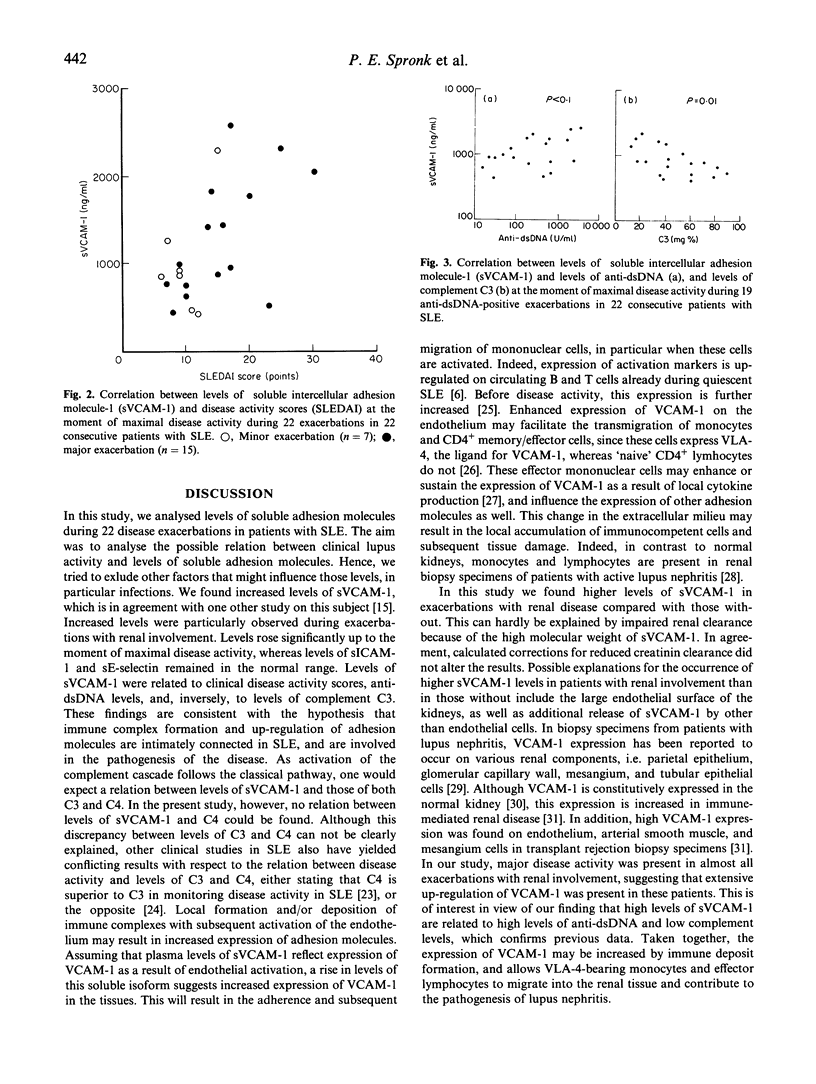
Abstract
Active SLE is characterized by immune deposits and subsequent vascular inflammation in many organs. Expression and up-regulation of adhesion molecules is basic to migration of inflammatory cells into the tissues. Recently, soluble isoforms of these molecules have been described which might be an expression of their up-regulation in the tissues and, as such, of disease activity. The purpose of this study was to evaluate whether changes in levels of soluble adhesion molecules reflect disease activity. We analysed serial sera in a 6-month period preceding 22 consecutive exacerbations of SLE for levels of soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), and sE-selectin. Levels were related to clinical disease activity (SLEDAI), and levels of anti-dsDNA and complement. At the time of maximal disease activity, levels of sVCAM-1 in patients with SLE were higher than those in controls (P < 0.0001), levels in patients with renal involvement being higher than in those without (P < 0.02). Levels of sVCAM-1 correlated with SLEDAI scores (P < 0.05) and, inversely, with levels of C3 (P = 0.01). In addition, in the presence of anti-dsDNA, levels of sVCAM-1 tended to correlate with levels of these autoantibodies (P < 0.1). Levels of sICAM-1 were normal and sE-selectin levels even decreased compared with controls. Levels of sVCAM-1 were higher at the moment of relapse (P = 0.001) than at 6 months before this time point. This rise correlated with the rise in SLEDAI score (P < 0.02). Levels of sICAM-1 and sE-selectin did not rise, and remained in the normal range in all exacerbations studied. In conclusion, in contrast to sICAM-1 and sE-selectin, levels of sVCAM-1 are increased, rise parallel to disease activity during exacerbations in SLE, and are associated with decreasing levels of complement factors. This favours the hypothesis of immune deposit formation, activation of the complement cascade and activation of endothelial cells. Concurrent up-regulation of vascular adhesion molecules may thus result in transmigration of activated inflammatory cells inducing tissue damage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers C. E., Hudkins K. L., Davis C. L., Marsh C. L., Riches W., McCarty J. M., Benjamin C. D., Carlos T. M., Harlan J. M., Lobb R. Expression of vascular cell adhesion molecule-1 in kidney allograft rejection. Kidney Int. 1993 Oct;44(4):805–816. doi: 10.1038/ki.1993.315. [DOI] [PubMed] [Google Scholar]
- Belmont H. M., Buyon J., Giorno R., Abramson S. Up-regulation of endothelial cell adhesion molecules characterizes disease activity in systemic lupus erythematosus. The Shwartzman phenomenon revisited. Arthritis Rheum. 1994 Mar;37(3):376–383. doi: 10.1002/art.1780370311. [DOI] [PubMed] [Google Scholar]
- Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Briscoe D. M., Cotran R. S., Pober J. S. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992 Nov 1;149(9):2954–2960. [PubMed] [Google Scholar]
- Bruijn J. A., Dinklo N. J. Distinct patterns of expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and endothelial-leukocyte adhesion molecule-1 in renal disease. Lab Invest. 1993 Sep;69(3):329–335. [PubMed] [Google Scholar]
- Carson C. W., Beall L. D., Hunder G. G., Johnson C. M., Newman W. Serum ELAM-1 is increased in vasculitis, scleroderma, and systemic lupus erythematosus. J Rheumatol. 1993 May;20(5):809–814. [PubMed] [Google Scholar]
- Cronstein B. N., Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993 Feb;36(2):147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- D'Agati V. D., Appel G. B., Estes D., Knowles D. M., 2nd, Pirani C. L. Monoclonal antibody identification of infiltrating mononuclear leukocytes in lupus nephritis. Kidney Int. 1986 Oct;30(4):573–581. doi: 10.1038/ki.1986.223. [DOI] [PubMed] [Google Scholar]
- Horgan K. J., Luce G. E., Tanaka Y., Schweighoffer T., Shimizu Y., Sharrow S. O., Shaw S. Differential expression of VLA-alpha 4 and VLA-beta 1 discriminates multiple subsets of CD4+CD45R0+ "memory" T cells. J Immunol. 1992 Dec 15;149(12):4082–4087. [PubMed] [Google Scholar]
- Lloyd W., Schur P. H. Immune complexes, complement, and anti-DNA in exacerbations of systemic lupus erythematosus (SLE). Medicine (Baltimore) 1981 May;60(3):208–217. doi: 10.1097/00005792-198105000-00004. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Chi-Rosso G., Leone D. R., Rosa M. D., Bixler S., Newman B. M., Luhowskyj S., Benjamin C. D., Dougas I. G., Goelz S. E. Expression and functional characterization of a soluble form of endothelial-leukocyte adhesion molecule 1. J Immunol. 1991 Jul 1;147(1):124–129. [PubMed] [Google Scholar]
- Machold K. P., Kiener H. P., Graninger W., Graninger W. B. Soluble intercellular adhesion molecule-1 (sICAM-1) in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1993 Jul;68(1):74–78. doi: 10.1006/clin.1993.1098. [DOI] [PubMed] [Google Scholar]
- Mason J. C., Kapahi P., Haskard D. O. Detection of increased levels of circulating intercellular adhesion molecule 1 in some patients with rheumatoid arthritis but not in patients with systemic lupus erythematosus. Lack of correlation with levels of circulating vascular cell adhesion molecule 1. Arthritis Rheum. 1993 Apr;36(4):519–527. doi: 10.1002/art.1780360412. [DOI] [PubMed] [Google Scholar]
- Orosz C. G., Ohye R. G., Pelletier R. P., Van Buskirk A. M., Huang E., Morgan C., Kincade P. W., Ferguson R. M. Treatment with anti-vascular cell adhesion molecule 1 monoclonal antibody induces long-term murine cardiac allograft acceptance. Transplantation. 1993 Aug;56(2):453–460. doi: 10.1097/00007890-199308000-00039. [DOI] [PubMed] [Google Scholar]
- Pallis M., Robson D. K., Haskard D. O., Powell R. J. Distribution of cell adhesion molecules in skeletal muscle from patients with systemic lupus erythematosus. Ann Rheum Dis. 1993 Sep;52(9):667–671. doi: 10.1136/ard.52.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott R., Needham L. A., Edwards R. M., Walker C., Power C. Structural and functional studies of the endothelial activation antigen endothelial leucocyte adhesion molecule-1 using a panel of monoclonal antibodies. J Immunol. 1991 Jul 1;147(1):130–135. [PubMed] [Google Scholar]
- Ricker D. M., Hebert L. A., Rohde R., Sedmak D. D., Lewis E. J., Clough J. D. Serum C3 levels are diagnostically more sensitive and specific for systemic lupus erythematosus activity than are serum C4 levels. The Lupus Nephritis Collaborative Study Group. Am J Kidney Dis. 1991 Dec;18(6):678–685. doi: 10.1016/s0272-6386(12)80609-3. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Mainolfi E. A., Czajkowski M., Marlin S. D. A form of circulating ICAM-1 in human serum. J Immunol. 1991 Dec 1;147(11):3788–3793. [PubMed] [Google Scholar]
- Seron D., Cameron J. S., Haskard D. O. Expression of VCAM-1 in the normal and diseased kidney. Nephrol Dial Transplant. 1991;6(12):917–922. doi: 10.1093/ndt/6.12.917. [DOI] [PubMed] [Google Scholar]
- Sfikakis P. P., Tesar J., Baraf H., Lipnick R., Klipple G., Tsokos G. C. Circulating intercellular adhesion molecule-1 in patients with systemic sclerosis. Clin Immunol Immunopathol. 1993 Jul;68(1):88–92. doi: 10.1006/clin.1993.1100. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Spronk P. E., vd Gun B. T., Limburg P. C., Kallenberg C. G. B cell activation in clinically quiescent systemic lupus erythematosus (SLE) is related to immunoglobulin levels, but not to levels of anti-dsDNA, nor to concurrent T cell activation. Clin Exp Immunol. 1993 Jul;93(1):39–44. doi: 10.1111/j.1365-2249.1993.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Verdegaal E. M., Beekhuizen H., Blokland I., van Furth R. Increased adhesion of human monocytes to IL-4-stimulated human venous endothelial cells via CD11/CD18, and very late antigen-4 (VLA-4)/vascular cell adhesion molecule-1 (VCAM-1)-dependent mechanisms. Clin Exp Immunol. 1993 Aug;93(2):292–298. doi: 10.1111/j.1365-2249.1993.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellicome S. M., Kapahi P., Mason J. C., Lebranchu Y., Yarwood H., Haskard D. O. Detection of a circulating form of vascular cell adhesion molecule-1: raised levels in rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol. 1993 Jun;92(3):412–418. doi: 10.1111/j.1365-2249.1993.tb03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcup S. M., DeBarge L. R., Caspi R. R., Harning R., Nussenblatt R. B., Chan C. C. Monoclonal antibodies against ICAM-1 (CD54) and LFA-1 (CD11a/CD18) inhibit experimental autoimmune uveitis. Clin Immunol Immunopathol. 1993 May;67(2):143–150. doi: 10.1006/clin.1993.1057. [DOI] [PubMed] [Google Scholar]
- Wuthrich R. P., Jevnikar A. M., Takei F., Glimcher L. H., Kelley V. E. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am J Pathol. 1990 Feb;136(2):441–450. [PMC free article] [PubMed] [Google Scholar]
- Wuthrich R. P. Vascular cell adhesion molecule-1 (VCAM-1) expression in murine lupus nephritis. Kidney Int. 1992 Oct;42(4):903–914. doi: 10.1038/ki.1992.367. [DOI] [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Hummel E. J., Limburg P. C., Kallenberg C. G. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990 May;33(5):634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]



