Abstract
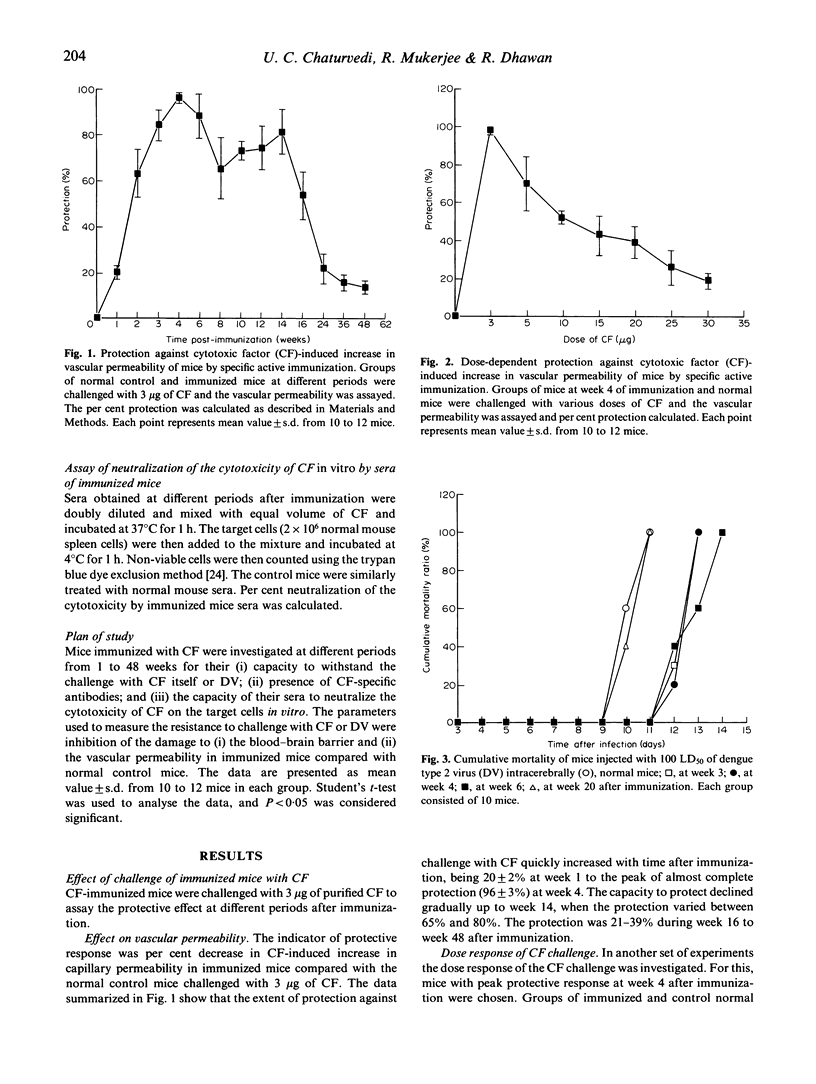
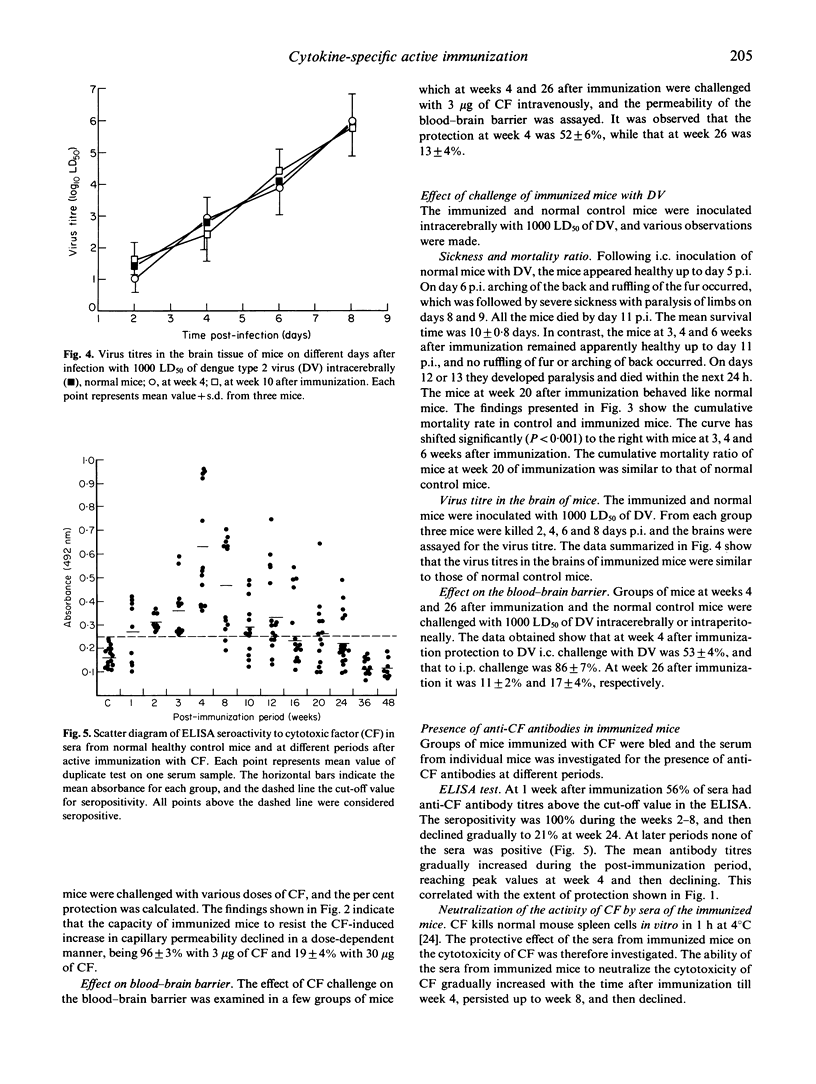
Dengue type 2 virus (DV)-induced cytotoxic factor (CF) is capable of reproducing various pathological lesions in mice that are seen in human dengue. The present study was undertaken to investigate the protective effect of active immunization of mice with CF. Mice were immunized with 5 microgram of CF and prevention of CF-induced increase in capillary permeability and damage to the blood-brain barrier were studied at weekly intervals, up to 48 weeks, by challenging with 3 microgram of CF. Maximum protection against increase in capillary permeability and damage to the blood-brain barrier was observed in week 4 after immunization. A breakthrough in the protection occurred with higher doses of CF in a dose-dependent manner. Challenge with a lethal intracerebral (i.c.) dose of DV showed significantly prolonged mean survival time and delayed onset of symptoms of sickness in the immunized mice compared with the normal mice, but the titre of the virus in the brain was similar in the two groups. On i.p. challenge with the virus the protection against damage to the blood-brain barrier was 86 +/- 7% at week 4 and 17 +/- 4% at week 26 after immunization. Sera obtained from the immunized mice showed the presence of CF-specific antibodies by ELISA, Western blot, and by neutralization of the cytotoxic activity of CF in vitro. The present study describes successful prevention of a cytokine-induced pathology by specific active immunization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendtzen K., Svenson M., Jønsson V., Hippe E. Autoantibodies to cytokines--friends or foes? Immunol Today. 1990 May;11(5):167–169. doi: 10.1016/0167-5699(90)90068-k. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Bhargava A., Mathur A. Production of cytotoxic factor in the spleen of dengue virus-infected mice. Immunology. 1980 Aug;40(4):665–671. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Dhawan R., Khanna M., Mathur A. Breakdown of the blood-brain barrier during dengue virus infection of mice. J Gen Virol. 1991 Apr;72(Pt 4):859–866. doi: 10.1099/0022-1317-72-4-859. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Nagar R., Gulati L., Mathur A. Variable effects of dengue virus-induced cytotoxic factors on different subpopulations of macrophages. Immunology. 1987 Jul;61(3):297–301. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Nagar R., Mathur A. Effect of dengue virus infection on Fc-receptor functions of mouse macrophages. J Gen Virol. 1983 Nov;64(Pt 11):2399–2407. doi: 10.1099/0022-1317-64-11-2399. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A. Effect of immunosuppression on dengue virus infection in mice. J Gen Virol. 1977 Sep;36(3):449–458. doi: 10.1099/0022-1317-36-3-449. [DOI] [PubMed] [Google Scholar]
- Dhawan R., Chaturvedi U. C., Khanna M., Mathur A., Tekwani B. L., Pandey V. C., Rai R. N. Obligatory role of Ca2+ in the cytotoxic activity of dengue virus-induced cytotoxin. Int J Exp Pathol. 1991 Feb;72(1):31–39. [PMC free article] [PubMed] [Google Scholar]
- Dhawan R., Khanna M., Chaturvedi U. C., Mathur A. Effect of dengue virus-induced cytotoxin on capillary permeability. J Exp Pathol (Oxford) 1990 Feb;71(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988 Jan 29;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Hotta H., Murakami I., Miyasaki K., Takeda Y., Shirane H., Hotta S. Inoculation of dengue virus into nude mice. J Gen Virol. 1981 Jan;52(Pt 1):71–76. doi: 10.1099/0022-1317-52-1-71. [DOI] [PubMed] [Google Scholar]
- Khanna M., Chaturvedi U. C., Dhawan R., Tekwani B. L., Pandey V. C. Presence of Ca2+ is obligatory for the cytotoxic activity of dengue virus-induced cytotoxic factor. Immunology. 1991 Jan;72(1):73–78. [PMC free article] [PubMed] [Google Scholar]
- Khanna M., Chaturvedi U. C. Purification and amino-terminal sequence of the dengue virus-induced cytotoxic factor. Int J Exp Pathol. 1992 Feb;73(1):43–49. [PMC free article] [PubMed] [Google Scholar]
- Khanna M., Chaturvedi U. C., Sharma M. C., Pandey V. C., Mathur A. Increased capillary permeability mediated by a dengue virus-induced lymphokine. Immunology. 1990 Mar;69(3):449–453. [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991 Jan;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Cole G. A. Immunosuppression and experimental virus infection of the nervous system. Adv Virus Res. 1970;16:397–448. doi: 10.1016/S0065-3527(08)60028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Taverne J., Bate C. A., de Souza J. B. The malaria vaccine: anti-parasite or anti-disease? Immunol Today. 1990 Jan;11(1):25–27. doi: 10.1016/0167-5699(90)90007-v. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wells R. A., Scott R. M., Pavanand K., Sathitsathein V., Cheamudon U., Macdermott R. P. Kinetics of peripheral blood leukocyte alterations in Thai children with dengue hemorrhagic fever. Infect Immun. 1980 May;28(2):428–433. doi: 10.1128/iai.28.2.428-433.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman D. R., McManus A. T., Eddy G. A. Extension of the mean time to death of mice with a lethal infection of Venezuelan equine encephalomyelitis virus by antithymocyte serum treatment. Infect Immun. 1975 Nov;12(5):1006–1011. doi: 10.1128/iai.12.5.1006-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Giovine F. S., Duff G. W. Interleukin 1: the first interleukin. Immunol Today. 1990 Jan;11(1):13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]


