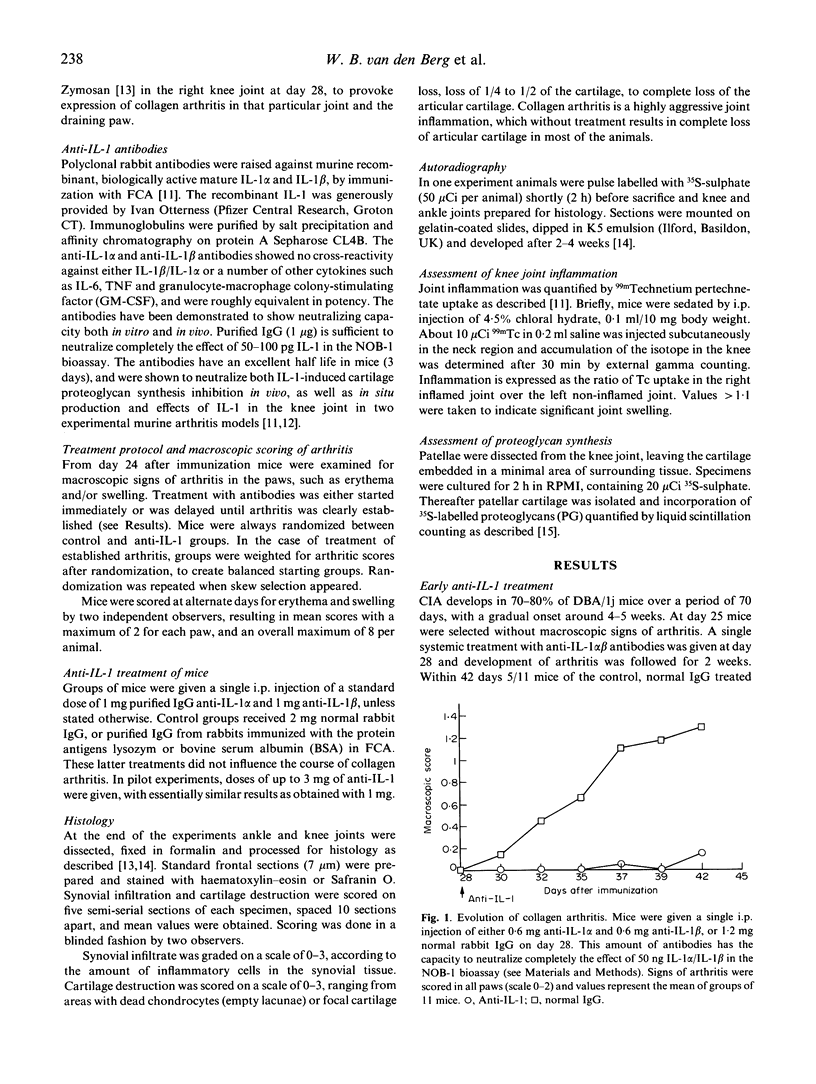
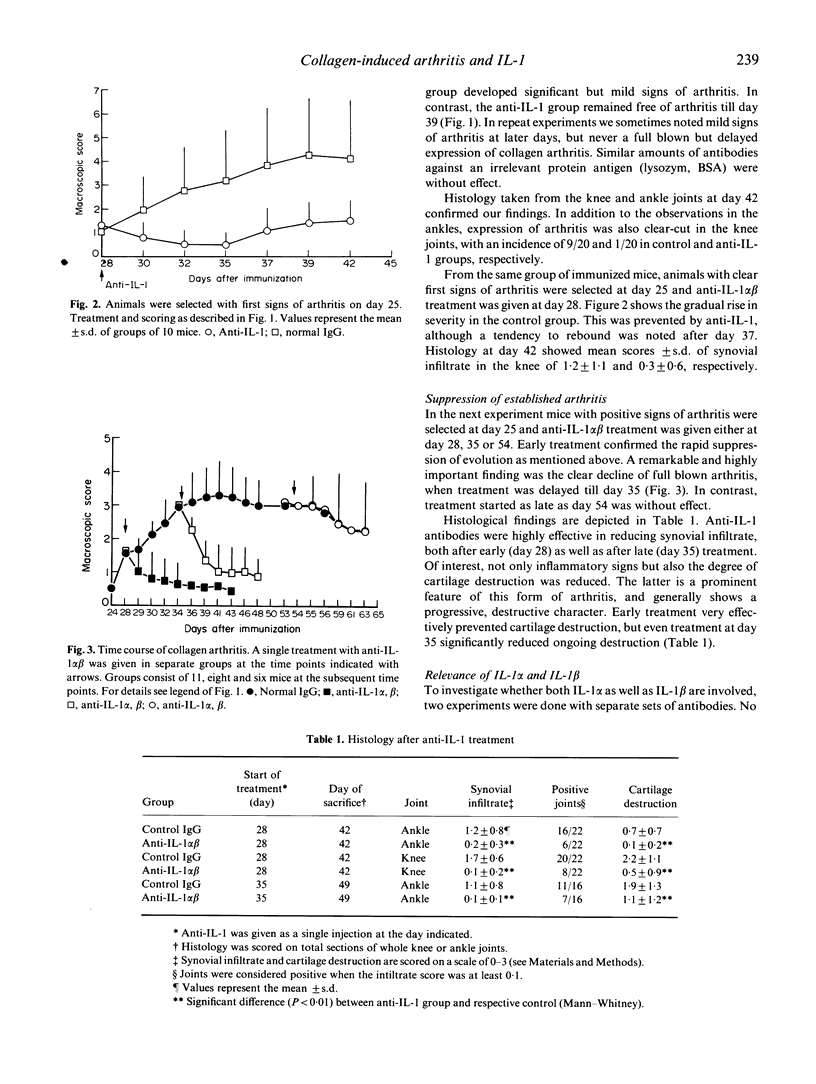
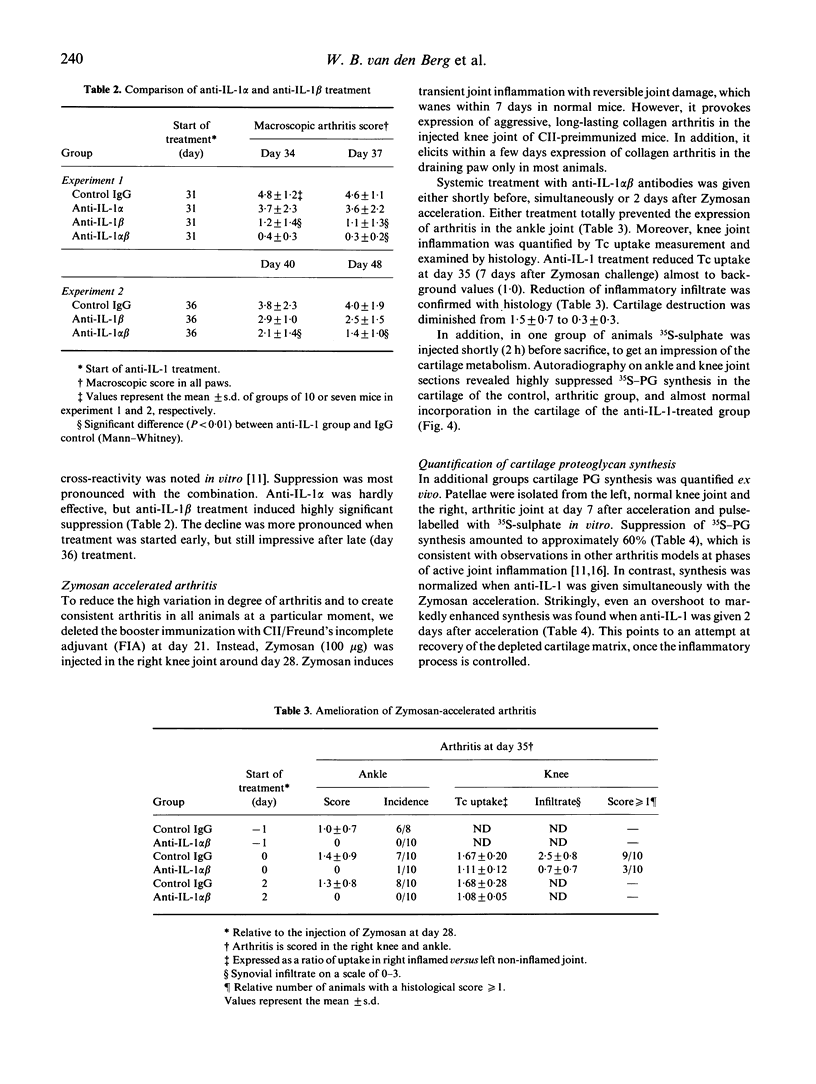
Abstract
Inflammatory cytokines have been implicated in the pathogenesis of rheumatoid arthritis. To validate a key role for IL-1 in arthritic processes we have studied the protective effect of neutralizing antimurine IL-1 antibodies in the murine collagen-induced arthritis (CIA) model. Combination of anti-IL-1 alpha and anti-IL-1 beta given before onset of arthritis was shown to prevent disease completely. Remarkably, a single treatment was also highly effective in the established phase of arthritis, reducing both inflammation as well as cartilage destruction. Suppression was most pronounced with the combination, but anti-IL-1 beta alone also induced significant relief. Finally, we studied the protective effect of IL-1 neutralization on cartilage metabolism in a unilateral expression model of collagen arthritis. To this end zymosan was injected in one knee joint before onset of disease, resulting in accelerated expression in that particular joint and the draining paw. Anti-IL-1 treatment started after accelerated expression of arthritis was able to fully normalize chondrocyte synthetic function, which was highly suppressed in the control group. It is concluded that IL-1 is an important determinant in both inflammation and cartilage destruction in collagen arthritis, and this may have implications for therapy in human arthritis.
Full text
PDF
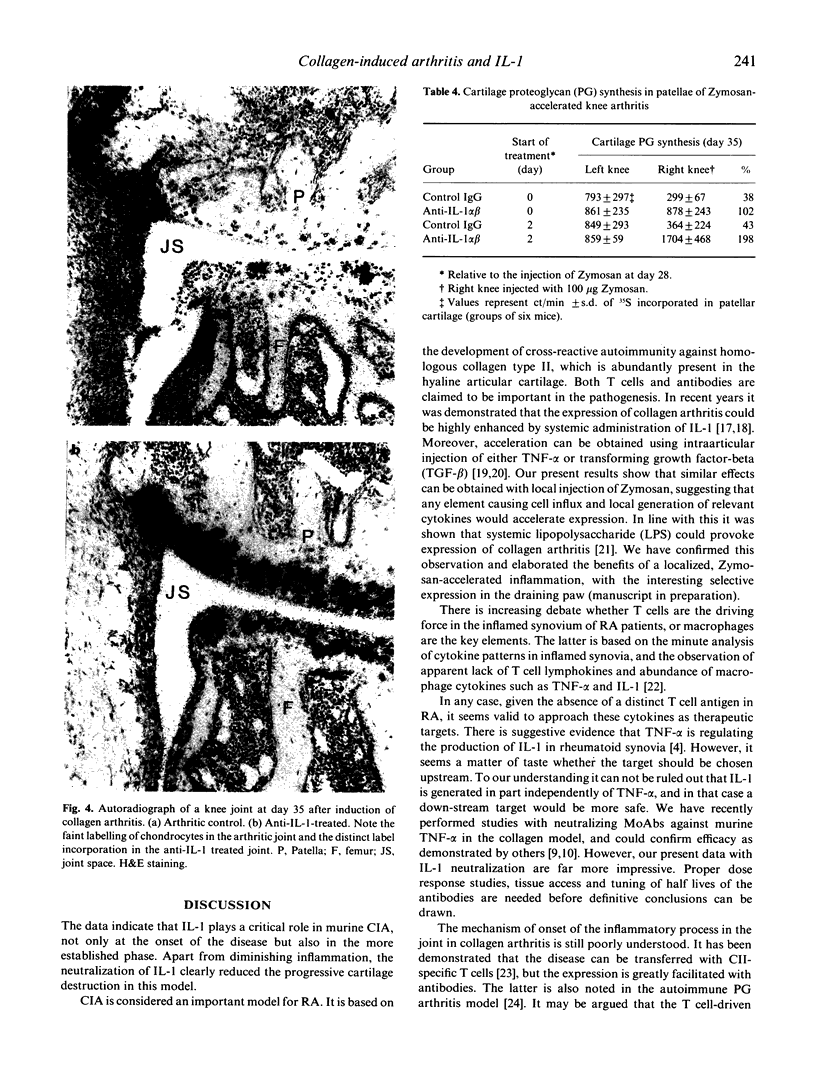


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro-Gracia J. M., Zvaifler N. J., Brown C. B., Kaushansky K., Firestein G. S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991 May 15;146(10):3365–3371. [PubMed] [Google Scholar]
- Arend W. P. IL-1 antagonism in inflammatory arthritis. Lancet. 1993 Jan 16;341(8838):155–156. doi: 10.1016/0140-6736(93)90014-8. [DOI] [PubMed] [Google Scholar]
- Brahn E., Trentham D. E. Experimental synovitis induced by collagen-specific T cell lines. Cell Immunol. 1989 Feb;118(2):491–503. doi: 10.1016/0008-8749(89)90396-1. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol. 1992 Dec;4(6):754–759. doi: 10.1016/0952-7915(92)90057-l. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Weiner H. L. T-cell vaccination. Immunol Today. 1988 Nov;9(11):332–335. doi: 10.1016/0167-5699(88)91330-8. [DOI] [PubMed] [Google Scholar]
- Cooper W. O., Fava R. A., Gates C. A., Cremer M. A., Townes A. S. Acceleration of onset of collagen-induced arthritis by intra-articular injection of tumour necrosis factor or transforming growth factor-beta. Clin Exp Immunol. 1992 Aug;89(2):244–250. doi: 10.1111/j.1365-2249.1992.tb06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Fenner H. The role of cytokines and their inhibitors in arthritis. Baillieres Clin Rheumatol. 1992 Jun;6(2):485–516. doi: 10.1016/s0950-3579(05)80186-4. [DOI] [PubMed] [Google Scholar]
- Hannum C. H., Wilcox C. J., Arend W. P., Joslin F. G., Dripps D. J., Heimdal P. L., Armes L. G., Sommer A., Eisenberg S. P., Thompson R. C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990 Jan 25;343(6256):336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hom J. T., Bendele A. M., Carlson D. G. In vivo administration with IL-1 accelerates the development of collagen-induced arthritis in mice. J Immunol. 1988 Aug 1;141(3):834–841. [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L. M., Dunn C. J. Interleukin-1 potentiates the development of collagen-induced arthritis in mice. Clin Sci (Lond) 1989 May;76(5):535–538. doi: 10.1042/cs0760535. [DOI] [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B. Influence of the severity and duration of murine antigen-induced arthritis on cartilage proteoglycan synthesis and chondrocyte death. Arthritis Rheum. 1985 Jul;28(7):813–819. doi: 10.1002/art.1780280713. [DOI] [PubMed] [Google Scholar]
- McIntyre K. W., Stepan G. J., Kolinsky K. D., Benjamin W. R., Plocinski J. M., Kaffka K. L., Campen C. A., Chizzonite R. A., Kilian P. L. Inhibition of interleukin 1 (IL-1) binding and bioactivity in vitro and modulation of acute inflammation in vivo by IL-1 receptor antagonist and anti-IL-1 receptor monoclonal antibody. J Exp Med. 1991 Apr 1;173(4):931–939. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikecz K., Glant T. T., Poole A. R. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987 Mar;30(3):306–318. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Vesin C., Loetscher H., Gentz R., Lesslauer W. Evolution of collagen arthritis in mice is arrested by treatment with anti-tumour necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. Immunology. 1992 Dec;77(4):510–514. [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Anderle S. K., Brown R. R., Dalldorf F. G., Thompson R. C. Pro- and anti-inflammatory roles of interleukin-1 in recurrence of bacterial cell wall-induced arthritis in rats. Infect Immun. 1991 Dec;59(12):4436–4442. doi: 10.1128/iai.59.12.4436-4442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Dalldorf F. G., Otterness I. G., Schwab J. H. Exacerbation of arthritis by IL-1 in rat joints previously injured by peptidoglycan-polysaccharide. J Immunol. 1988 May 1;140(9):2964–2969. [PubMed] [Google Scholar]
- Thorbecke G. J., Shah R., Leu C. H., Kuruvilla A. P., Hardison A. M., Palladino M. A. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. O., Feldmann M., Maini R. N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B. Impact of NSAIDS on murine antigen induced arthritis. I. Investigation of antiinflammatory and chondroprotective effects. J Rheumatol Suppl. 1989 Aug;18:10–18. [PubMed] [Google Scholar]
- van Beuningen H. M., Arntz O. J., van den Berg W. B. In vivo effects of interleukin-1 on articular cartilage. Prolongation of proteoglycan metabolic disturbances in old mice. Arthritis Rheum. 1991 May;34(5):606–615. doi: 10.1002/art.1780340513. [DOI] [PubMed] [Google Scholar]
- van Lent P. L., van den Bersselaar L. A., van den Hoek A. E., van de Loo A. A., van den Berg W. B. Cationic immune complex arthritis in mice--a new model. Synergistic effect of complement and interleukin-1. Am J Pathol. 1992 Jun;140(6):1451–1461. [PMC free article] [PubMed] [Google Scholar]
- van de Loo A. A., Arntz O. J., van den Berg W. B. Flare-up of experimental arthritis in mice with murine recombinant IL-1. Clin Exp Immunol. 1992 Feb;87(2):196–202. doi: 10.1111/j.1365-2249.1992.tb02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo A. A., van den Berg W. B. Effects of murine recombinant interleukin 1 on synovial joints in mice: measurement of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis. 1990 Apr;49(4):238–245. doi: 10.1136/ard.49.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo F. A., Arntz O. J., Otterness I. G., van den Berg W. B. Protection against cartilage proteoglycan synthesis inhibition by antiinterleukin 1 antibodies in experimental arthritis. J Rheumatol. 1992 Mar;19(3):348–356. [PubMed] [Google Scholar]
- van den Berg W. B., Kruijsen M. W., van de Putte L. B., van Beusekom H. J., van der Sluis-van der Pol M., Zwarts W. A. Antigen-induced and zymosan-induced arthritis in mice: studies on in vivo cartilage proteoglycan synthesis and chondrocyte death. Br J Exp Pathol. 1981 Jun;62(3):308–316. [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., van Beusekom H. J., van de Putte L. B., Zwarts W. A., van der Sluis M. Antigen handling in antigen-induced arthritis in mice: an autoradiographic and immunofluorescence study using whole joint sections. Am J Pathol. 1982 Jul;108(1):9–16. [PMC free article] [PubMed] [Google Scholar]