Abstract
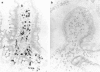
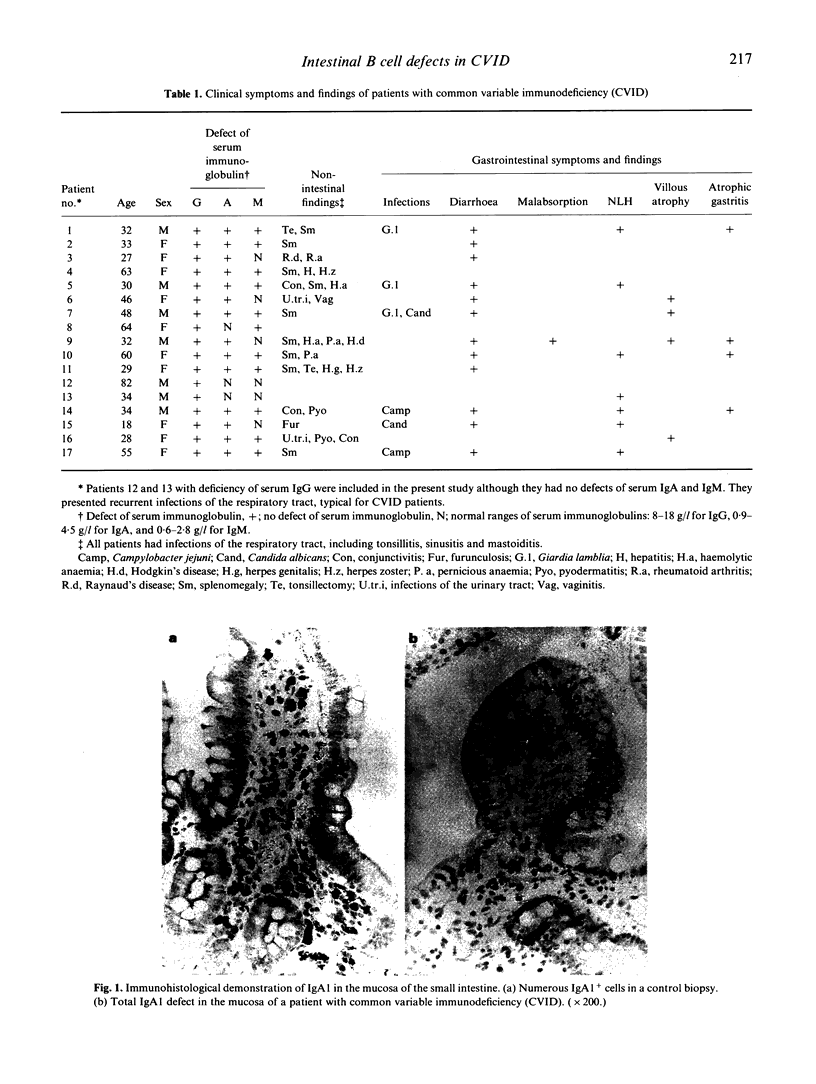
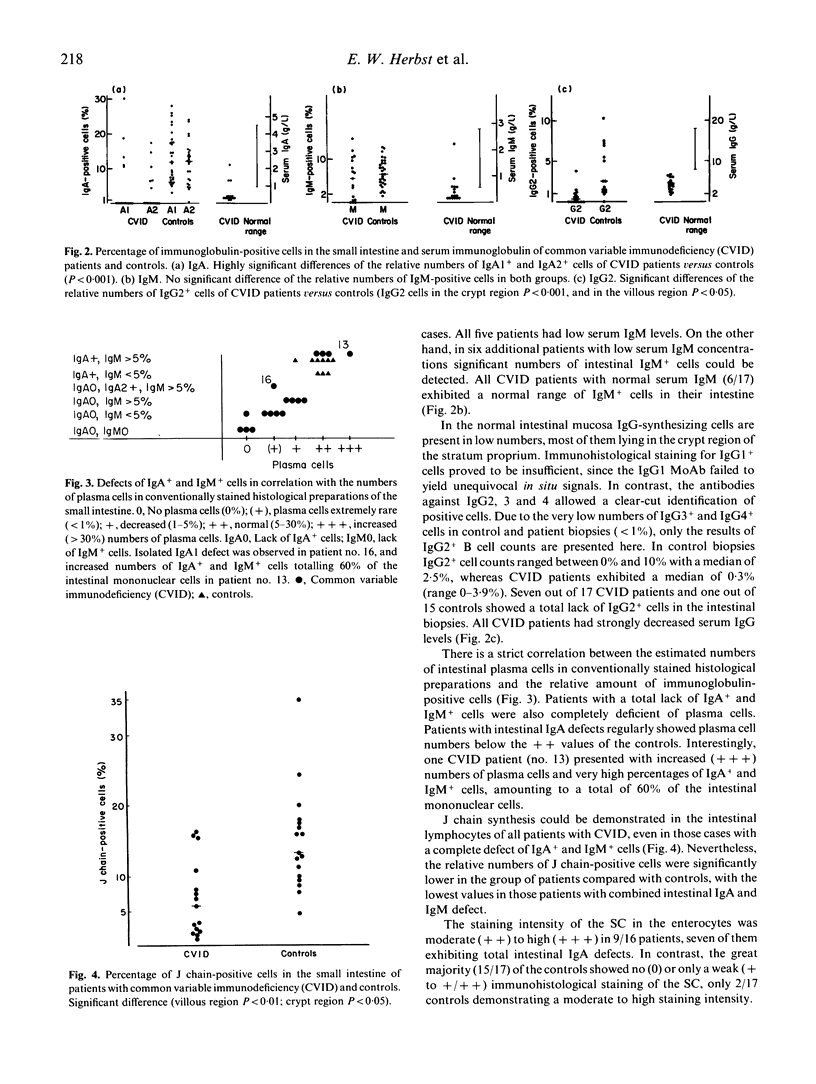
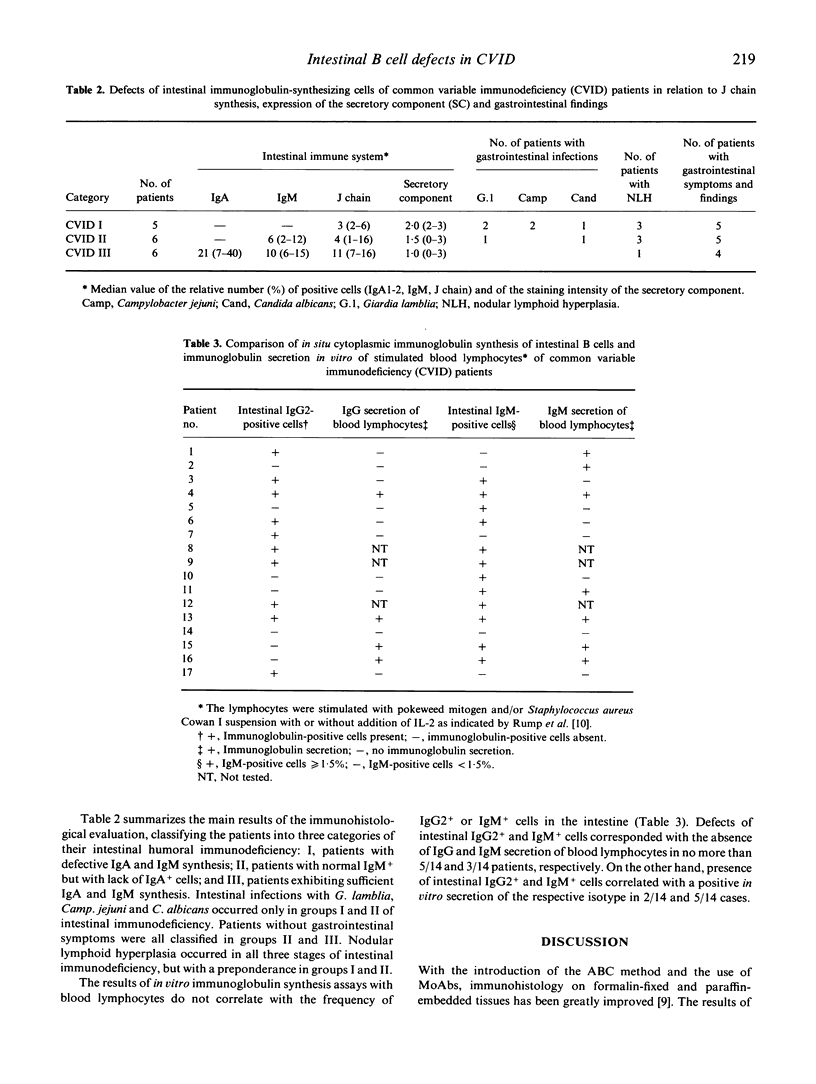
The humoral immune system of the small intestine of 17 patients with common variable immunodeficiency (CVID) was studied by immunohistology using antibodies specific for IgA1,2, IgM, IgG1-4, the J chain and the secretory component (SC). IgA1,2+, IgG2+ and IgM+ lamina propria B cells were totally lacking in 65% (11/17), 41% (7/17) and 18% (3/17) of CVID patients, respectively. One patient exhibited an isolated IgA1 subclass deficiency. The proportion of plasma cells in conventionally stained histological sections of the same intestinal biopsies showed a close correlation with the numbers of IgA+ and IgM+ cells. Considerable numbers of J chain-synthesizing cells were present in all patients with CVID, indicating the presence of early B cells unable to differentiate into immunoglobulin-producing plasma cells. Most of the patients with intestinal IgA and/or IgM defects strongly expressed the SC in their enterocytes, suggesting an immunoglobulin-independent regulation of the SC. Clinically, only CVID patients with intestinal IgA defects developed intestinal infections with Giardia lamblia, Campylobacter jejuni or Candida albicans. The outcome of in vitro immunoglobulin synthesis assays with peripheral blood lymphocytes did not predict the presence or absence of the respective isotype-producing B cells in the intestinal lamina propria. Thus, immunohistological examinations of intestinal biopsies are required to determine the extent of mucosal immunodeficiency in CVID patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Brown W. R., Kloppel T. M. Secretory component: the polymeric immunoglobulin receptor. What's in it for the gastroenterologist and hepatologist? Gastroenterology. 1985 Sep;89(3):667–682. doi: 10.1016/0016-5085(85)90467-6. [DOI] [PubMed] [Google Scholar]
- Baumert E., Wolff-Vorbeck G., Schlesier M., Peter H. H. Immunophenotypical alterations in a subset of patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 1992 Oct;90(1):25–30. doi: 10.1111/j.1365-2249.1992.tb05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Korsrud F. R. Significance of different J chain profiles in human tissues: generation of IgA and IgM with binding site for secretory component is related to the J chain expressing capacity of the total local immunocyte population, including IgG and IgD producing cells, and depends on the clinical state of the tissue. Clin Exp Immunol. 1984 Dec;58(3):709–718. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Nilssen D. E., Rognum T. O., Thrane P. S. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am. 1991 Sep;20(3):397–439. [PubMed] [Google Scholar]
- Broom B. C., de la Concha E. G., Webster A. D., Loewi G., Asherson G. L. Dichotomy between immunoglobulin synthesis by cells in gut and blood of patients with hypogammaglobulinaemia. Lancet. 1975 Aug 9;2(7928):253–256. doi: 10.1016/s0140-6736(75)90965-4. [DOI] [PubMed] [Google Scholar]
- Bryant A., Calver N. C., Toubi E., Webster A. D., Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990 Aug;56(2):239–248. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- Bästlein C., Burlefinger R., Holzberg E., Voeth C., Garbrecht M., Ottenjann R. Common variable immunodeficiency syndrome and nodular lymphoid hyperplasia in the small intestine. Endoscopy. 1988 Sep;20(5):272–275. doi: 10.1055/s-2007-1018192. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989 Jan;9(1):22–33. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- Dura W. T., Bernatowska E. Secretory component, alpha 1-antitrypsin and lysozyme in IgA deficient children. An immunohistochemical evaluation of intestinal mucosa. Histopathology. 1984 Sep;8(5):747–757. doi: 10.1111/j.1365-2559.1984.tb02391.x. [DOI] [PubMed] [Google Scholar]
- Fiedler W., Sykora K. W., Welte K., Kolitz J. E., Cunningham-Rundles C., Holloway K., Miller G. A., Souza L., Mertelsmann R. T-cell activation defect in common variable immunodeficiency: restoration by phorbol myristate acetate (PMA) or allogeneic macrophages. Clin Immunol Immunopathol. 1987 Aug;44(2):206–218. doi: 10.1016/0090-1229(87)90066-3. [DOI] [PubMed] [Google Scholar]
- Hermans P. E., Diaz-Buxo J. A., Stobo J. D. Idiopathic late-onset immunoglobulin deficiency. Clinical observations in 50 patients. Am J Med. 1976 Aug;61(2):221–237. doi: 10.1016/0002-9343(76)90173-x. [DOI] [PubMed] [Google Scholar]
- Kelényi G. Intracellular J chains in lymphoproliferative diseases. Virchows Arch A Pathol Anat Histopathol. 1985;405(3):365–378. doi: 10.1007/BF00710071. [DOI] [PubMed] [Google Scholar]
- Kinlen L. J., Webster A. D., Bird A. G., Haile R., Peto J., Soothill J. F., Thompson R. A. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985 Feb 2;1(8423):263–266. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- Koshland M. E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Lebranchu Y., Thibault G., Degenne D., Bardos P. Abnormalities in CD4+ T lymphocyte subsets in patients with common variable immunodeficiency. Clin Immunol Immunopathol. 1991 Oct;61(1):83–92. doi: 10.1016/s0090-1229(06)80009-7. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Russell M. W. IgA subclasses. Monogr Allergy. 1986;19:277–301. [PubMed] [Google Scholar]
- Nagura H., Kohler P. F., Brown W. R. Immunocytochemical characterization of the lymphocytes in nodular lymphoid hyperplasia of the bowel. Lab Invest. 1979 Jan;40(1):66–73. [PubMed] [Google Scholar]
- Norton A. J., Isaacson P. G. Detailed phenotypic analysis of B-cell lymphoma using a panel of antibodies reactive in routinely fixed wax-embedded tissue. Am J Pathol. 1987 Aug;128(2):225–240. [PMC free article] [PubMed] [Google Scholar]
- Rump J. A., Jahreis A., Schlesier M., Dräger R., Melchers I., Peter H. H. Possible role of IL-2 deficiency for hypogammaglobulinaemia in patients with common variable immunodeficiency. Clin Exp Immunol. 1992 Aug;89(2):204–210. doi: 10.1111/j.1365-2249.1992.tb06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Giorgi J. V., Sherr E. H., Kagan J. M. Failure of B cells in common variable immunodeficiency to transit from proliferation to differentiation is associated with altered B cell surface-molecule display. J Allergy Clin Immunol. 1989 Jul;84(1):44–55. doi: 10.1016/0091-6749(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Sneller M. C., Strober W. Abnormalities of lymphokine gene expression in patients with common variable immunodeficiency. J Immunol. 1990 May 15;144(10):3762–3769. [PubMed] [Google Scholar]
- Underdown B. J., Schiff J. M. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- Van den Brande P., Geboes K., Vantrappen G., Van den Eeckhout A., Vertessen S., Stevens E. A., Ceuppens J. L. Intestinal nodular lymphoid hyperplasia in patients with common variable immunodeficiency: local accumulation of B and CD8(+) lymphocytes. J Clin Immunol. 1988 Jul;8(4):296–306. doi: 10.1007/BF00916558. [DOI] [PubMed] [Google Scholar]
- Wright J. J., Wagner D. K., Blaese R. M., Hagengruber C., Waldmann T. A., Fleisher T. A. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990 Nov 15;76(10):2046–2051. [PubMed] [Google Scholar]