Abstract
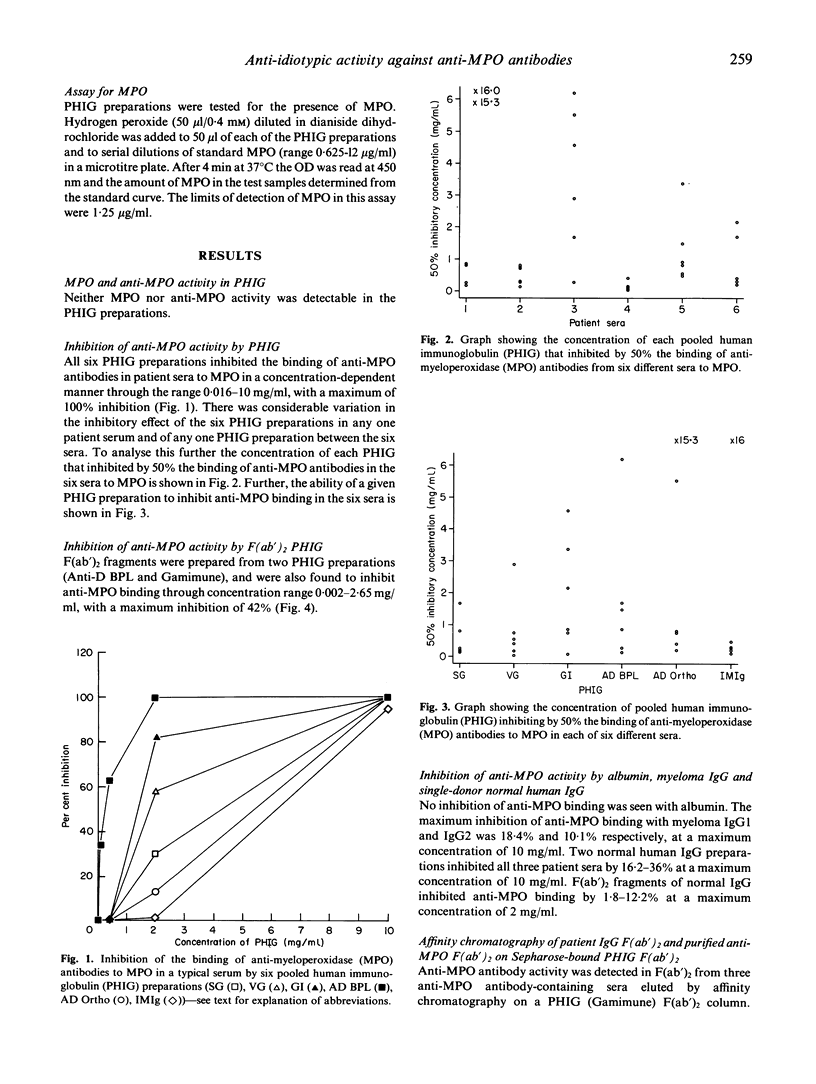
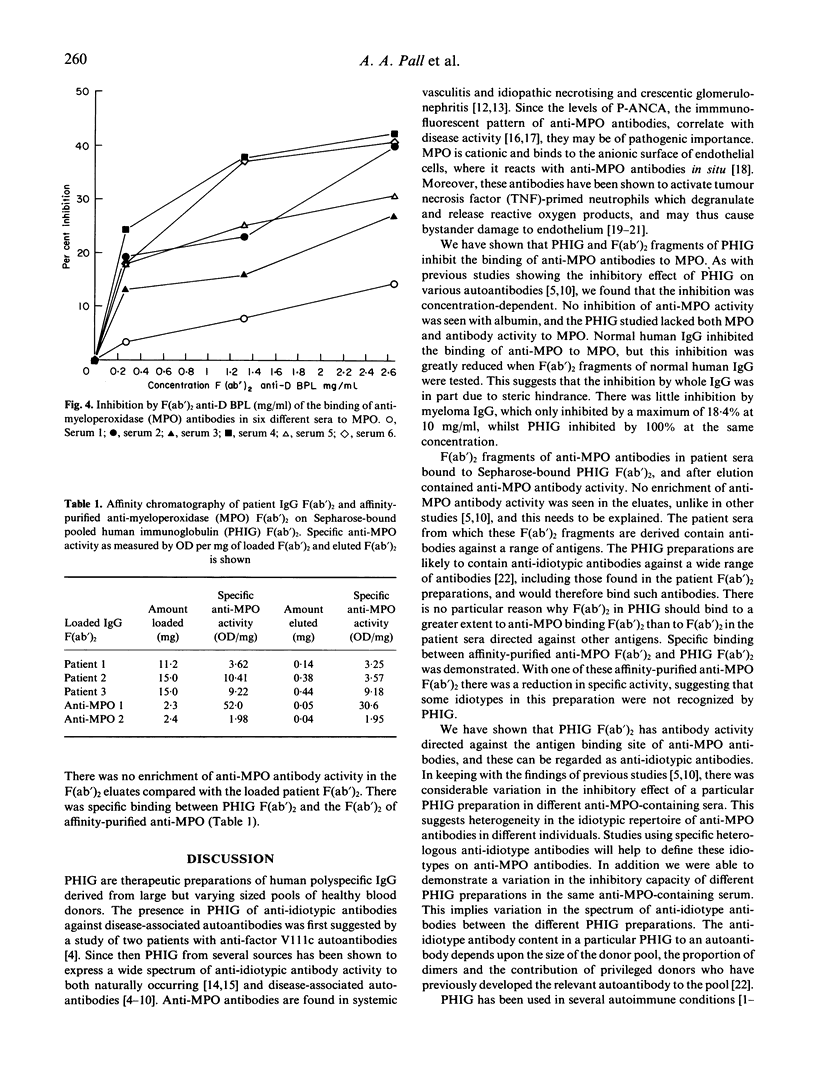
We investigated the ability of six different pooled human immunoglobulin (PHIG) preparations to inhibit the binding of anti-myeloperoxidase (MPO) antibodies to MPO. All six PHIG preparations inhibited the binding of anti-MPO antibodies from six sera to MPO in a concentration-dependent manner in the concentration range 0.016-10 mg/ml. There was considerable variation in the ability of each PHIG preparation to inhibit the binding of anti-MPO antibody in a given serum. Further differences were seen in the ability of a given PHIG to inhibit anti-MPO binding in different sera. F(ab')2 fragments from two PHIG preparations also inhibited in a concentration-dependent manner anti-MPO binding to MPO in all six sera in the concentration range 0.002-2.65 mg/ml, with a maximum inhibition of 42%. Little inhibition was seen with F(ab')2 of normal human IgG from individual donors (1.8-12.2% at the maximum concentration of 2 mg/ml). F(ab')2 fragments from three anti-MPO containing sera and two affinity-purified anti-MPO antibodies were eluted by affinity chromatography from Sepharose-bound PHIG F(ab')2 and showed anti-MPO antibody activity. We have shown that PHIG and F(ab')2 fragments of PHIG inhibit anti-MPO binding to MPO, and further that F(ab')2 fragments of PHIG bind to F(ab')2 fragments of anti-MPO antibodies. These observations indicate binding between the variable regions of PHIG and the antigen binding site of anti-MPO antibodies, and are consistent with an anti-idiotypic reaction. The variability seen in the inhibitory effect of the different PHIG preparations in anti-MPO-positive sera implies differences in their anti-idiotype content, while the variability of the inhibitory effect of a particular PHIG preparation between different sera suggests heterogeneity in the idiotypic repertoire of anti-MPO antibodies. Such variations in the inhibitory effect of different PHIG preparations on antibody binding may be an important determinant of their therapeutic effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballow M. Mechanisms of action of intravenous immunoglobulin therapy and potential use in autoimmune connective tissue diseases. Cancer. 1991 Sep 15;68(6 Suppl):1430–1436. doi: 10.1002/1097-0142(19910915)68:6+<1430::aid-cncr2820681405>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Ballow M., White W., Desbonnet C. Modulation of in vitro synthesis of immunoglobulin and the induction of suppressor activity by therapy with intravenous immune globulin. J Allergy Clin Immunol. 1989 Oct;84(4 Pt 2):595–602. doi: 10.1016/0091-6749(89)90196-6. [DOI] [PubMed] [Google Scholar]
- Basta M., Kirshbom P., Frank M. M., Fries L. F. Mechanism of therapeutic effect of high-dose intravenous immunoglobulin. Attenuation of acute, complement-dependent immune damage in a guinea pig model. J Clin Invest. 1989 Dec;84(6):1974–1981. doi: 10.1172/JCI114387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold P., Dale G. L., Tani P., McMillan R. Inhibition of autoantibody binding to platelet glycoprotein IIb/IIIa by anti-idiotypic antibodies in intravenous gammaglobulin. Blood. 1989 Nov 15;74(7):2414–2417. [PubMed] [Google Scholar]
- Bussel J. B., Kimberly R. P., Inman R. D., Schulman I., Cunningham-Rundles C., Cheung N., Smithwick E. M., O'Malley J., Barandun S., Hilgartner M. W. Intravenous gammaglobulin treatment of chronic idiopathic thrombocytopenic purpura. Blood. 1983 Aug;62(2):480–486. [PubMed] [Google Scholar]
- Dietrich G., Algiman M., Sultan Y., Nydegger U. E., Kazatchkine M. D. Origin of anti-idiotypic activity against anti-factor VIII autoantibodies in pools of normal human immunoglobulin G (IVIg). Blood. 1992 Jun 1;79(11):2946–2951. [PubMed] [Google Scholar]
- Dietrich G., Kazatchkine M. D. Normal immunoglobulin G (IgG) for therapeutic use (intravenous Ig) contain antiidiotypic specificities against an immunodominant, disease-associated, cross-reactive idiotype of human anti-thyroglobulin autoantibodies. J Clin Invest. 1990 Mar;85(3):620–625. doi: 10.1172/JCI114483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner W., Chapel H. M. Titration of antibodies against neutrophil cytoplasmic antigens is useful in monitoring disease activity in systemic vasculitides. Clin Exp Immunol. 1990 Nov;82(2):244–249. doi: 10.1111/j.1365-2249.1990.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert B. H., Jennette J. C., Falk R. J. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992 Feb;41(2):375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988 Jun 23;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Terrell R. S., Charles L. A., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr J., Hofmann V., Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N Engl J Med. 1982 May 27;306(21):1254–1258. doi: 10.1056/NEJM198205273062102. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Viale A. C., Sundblad A., Heusser C., Coutinho A. Normal serum immunoglobulins participate in the selection of peripheral B-cell repertoires. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5640–5644. doi: 10.1073/pnas.88.13.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgartner M. W., Bussel J. Use of intravenous gamma globulin for the treatment of autoimmune neutropenia of childhood and autoimmune hemolytic anemia. Am J Med. 1987 Oct 23;83(4A):25–29. doi: 10.1016/0002-9343(87)90547-x. [DOI] [PubMed] [Google Scholar]
- Imbach P., Barandun S., d'Apuzzo V., Baumgartner C., Hirt A., Morell A., Rossi E., Schöni M., Vest M., Wagner H. P. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981 Jun 6;1(8232):1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- Imbach P., Wagner H. P., Berchtold W., Gaedicke G., Hirt A., Joller P., Mueller-Eckhardt C., Müller B., Rossi E., Barandun S. Intravenous immunoglobulin versus oral corticosteroids in acute immune thrombocytopenic purpura in childhood. Lancet. 1985 Aug 31;2(8453):464–468. doi: 10.1016/s0140-6736(85)90400-3. [DOI] [PubMed] [Google Scholar]
- Jayne D. R., Davies M. J., Fox C. J., Black C. M., Lockwood C. M. Treatment of systemic vasculitis with pooled intravenous immunoglobulin. Lancet. 1991 May 11;337(8750):1137–1139. doi: 10.1016/0140-6736(91)92797-6. [DOI] [PubMed] [Google Scholar]
- Kaveri S. V., Dietrich G., Hurez V., Kazatchkine M. D. Intravenous immunoglobulins (IVIg) in the treatment of autoimmune diseases. Clin Exp Immunol. 1991 Nov;86(2):192–198. doi: 10.1111/j.1365-2249.1991.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly R. P., Salmon J. E., Bussel J. B., Crow M. K., Hilgartner M. W. Modulation of mononuclear phagocyte function by intravenous gamma-globulin. J Immunol. 1984 Feb;132(2):745–750. [PubMed] [Google Scholar]
- Lee S. S., Adu D., Thompson R. A. Anti-myeloperoxidase antibodies in systemic vasculitis. Clin Exp Immunol. 1990 Jan;79(1):41–46. doi: 10.1111/j.1365-2249.1990.tb05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist I., van Doorn P. A., Vermeulen M., van Lint M., van Rood J. J., Brand A. Regulation of autoantibodies in inflammatory demyelinating polyneuropathy: spontaneous and therapeutic. Immunol Rev. 1989 Aug;110:105–117. doi: 10.1111/j.1600-065x.1989.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Newburger J. W., Takahashi M., Beiser A. S., Burns J. C., Bastian J., Chung K. J., Colan S. D., Duffy C. E., Fulton D. R., Glode M. P. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991 Jun 6;324(23):1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- Pettersson E., Heigl Z. Antineutrophil cytoplasmic antibody (cANCA and pANCA) titers in relation to disease activity in patients with necrotizing vasculitis: a longitudinal study. Clin Nephrol. 1992 May;37(5):219–228. [PubMed] [Google Scholar]
- Rossi F., Dietrich G., Kazatchkine M. D. Anti-idiotypes against autoantibodies in normal immunoglobulins: evidence for network regulation of human autoimmune responses. Immunol Rev. 1989 Aug;110:135–149. doi: 10.1111/j.1600-065x.1989.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Rossi F., Guilbert B., Tonnelle C., Ternynck T., Fumoux F., Avrameas S., Kazatchkine M. D. Idiotypic interactions between normal human polyspecific IgG and natural IgM antibodies. Eur J Immunol. 1990 Sep;20(9):2089–2094. doi: 10.1002/eji.1830200930. [DOI] [PubMed] [Google Scholar]
- Rossi F., Jayne D. R., Lockwood C. M., Kazatchkine M. D. Anti-idiotypes against anti-neutrophil cytoplasmic antigen autoantibodies in normal human polyspecific IgG for therapeutic use and in the remission sera of patients with systemic vasculitis. Clin Exp Immunol. 1991 Feb;83(2):298–303. doi: 10.1111/j.1365-2249.1991.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. O., Pottinger B. E., Gaskin G., Pusey C. D., Pearson J. D. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol. 1992 Aug;141(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Shimozato T., Iwata M., Kawada H., Tamura N. Human immunoglobulin preparation for intravenous use induces elevation of cellular cyclic adenosine 3':5'-monophosphate levels, resulting in suppression of tumour necrosis factor alpha and interleukin-1 production. Immunology. 1991 Apr;72(4):497–501. [PMC free article] [PubMed] [Google Scholar]
- Stohl W. Cellular mechanisms in the in vitro inhibition of pokeweed mitogen-induced B cell differentiation by immunoglobulin for intravenous use. J Immunol. 1986 Jun 15;136(12):4407–4413. [PubMed] [Google Scholar]
- Sultan Y., Kazatchkine M. D., Maisonneuve P., Nydegger U. E. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet. 1984 Oct 6;2(8406):765–768. doi: 10.1016/s0140-6736(84)90701-3. [DOI] [PubMed] [Google Scholar]
- Sundblad A., Huetz F., Portnoï D., Coutinho A. Stimulation of B and T cells by in vivo high dose immunoglobulin administration in normal mice. J Autoimmun. 1991 Apr;4(2):325–339. doi: 10.1016/0896-8411(91)90028-b. [DOI] [PubMed] [Google Scholar]
- Vargunam M., Adu D., Taylor C. M., Michael J., Richards N., Neuberger J., Thompson R. A. Endothelium myeloperoxidase-antimyeloperoxidase interaction in vasculitis. Nephrol Dial Transplant. 1992;7(11):1077–1081. [PubMed] [Google Scholar]
- van Doorn P. A., Brand A., Strengers P. F., Meulstee J., Vermeulen M. High-dose intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double-blind, placebo-controlled, crossover study. Neurology. 1990 Feb;40(2):209–212. doi: 10.1212/wnl.40.2.209. [DOI] [PubMed] [Google Scholar]


