Abstract
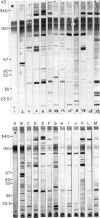
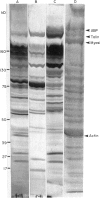
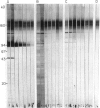
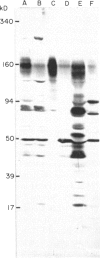
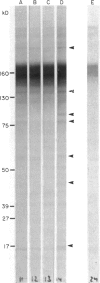
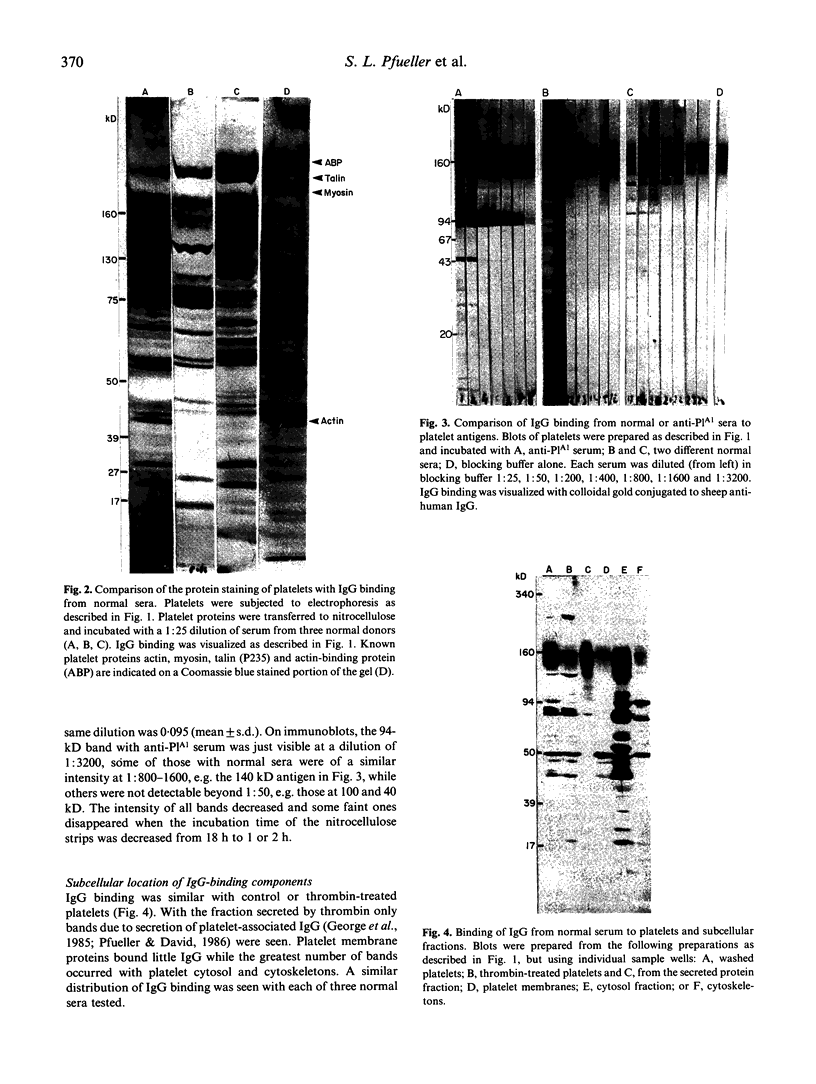
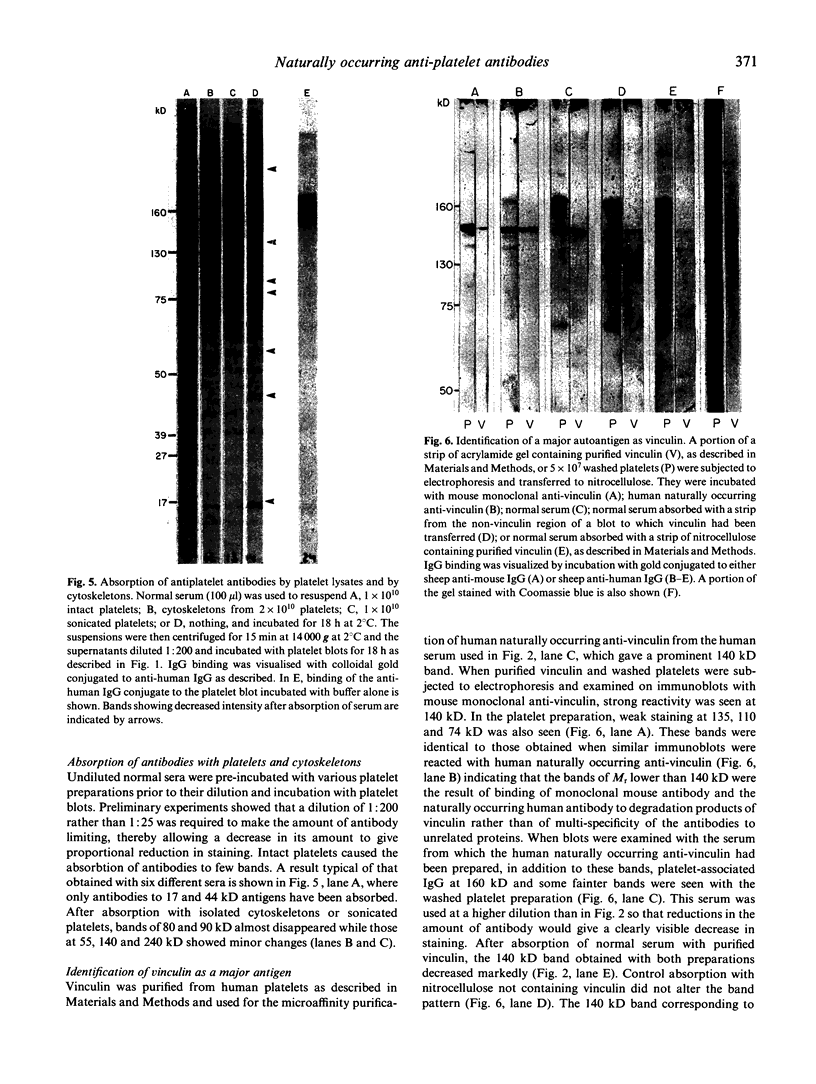
Immunoblotting of platelets that have been subjected to SDS-PAGE has revealed that sera from normal individuals contain IgG which binds to many platelet components. This binding was seen with autologous and heterologous platelets using serum of males and of nulliparous females who had not received blood transfusions. Although binding patterns of different sera were not identical, almost all sera caused IgG binding to platelet components of 87-90 kD, 140 kD (identified as vinculin) and 220-240 kD (tentatively identified as talin and actin-binding protein). Purified IgG showed the same binding pattern as whole serum and F(ab')2 fragments retained their ability to bind to many components. The titre of IgG binding in serum was 1:50-1600 while that of alloantibodies to the PlA1 antigen was 1:3200. IgG binding components were not secreted when platelets were stimulated and were rarely associated with isolated membranes, but were located either in platelet cytoplasm or cytoskeletons. IgG binding was decreased by absorbing sera with lysed platelets or isolated cytoskeletons, but only slightly with intact platelets. Microaffinity purification of IgG which formed a major band on immunoblots showed that it was antibody with specificity for vinculin or its degradation products. These findings suggest that normal sera contain naturally occurring IgG antibodies with specificity for intracellular platelet antigens and that in some cases their titre approaches that of antibodies of pathological significance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dighiero G., Guilbert B., Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982 Jun;128(6):2788–2792. [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Fermand J. P., Lymberi P., Danon F., Avrameas S. Thirty-six human monoclonal immunoglobulins with antibody activity against cytoskeleton proteins, thyroglobulin, and native DNA: immunologic studies and clinical correlations. Blood. 1983 Aug;62(2):264–270. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Feramisco J. R., Burridge K. A rapid purification of alpha-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980 Feb 10;255(3):1194–1199. [PubMed] [Google Scholar]
- Fox J. E. Linkage of a membrane skeleton to integral membrane glycoproteins in human platelets. Identification of one of the glycoproteins as glycoprotein Ib. J Clin Invest. 1985 Oct;76(4):1673–1683. doi: 10.1172/JCI112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Saucerman S., Levine S. P., Knieriem L. K., Bainton D. F. Immunoglobulin G is a platelet alpha granule-secreted protein. J Clin Invest. 1985 Nov;76(5):2020–2025. doi: 10.1172/JCI112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Grabar P. Hypothesis. Auto-antibodies and immunological theories: an analytical review. Clin Immunol Immunopathol. 1975 Nov;4(4):453–466. doi: 10.1016/0090-1229(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Hsu Y. H. Immunogold for detection of antigen on nitrocellulose paper. Anal Biochem. 1984 Oct;142(1):221–225. doi: 10.1016/0003-2697(84)90542-6. [DOI] [PubMed] [Google Scholar]
- Kaise S., Yasuda T., Kasukawa R., Nishimaki T., Watarai S., Tsumita T. Antiglycolipid antibodies in normal and pathologic human sera and synovial fluids. Vox Sang. 1985;49(4):292–300. doi: 10.1111/j.1423-0410.1985.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Kaplan C., Champeix P., Blanchard D., Muller J. Y., Cartron J. P. Platelet antibodies in systemic lupus erythematosus. Br J Haematol. 1987 Sep;67(1):89–93. doi: 10.1111/j.1365-2141.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981 Feb 5;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- McMillan R., Mason D., Tani P., Schmidt G. M. Evaluation of platelet surface antigens: localization of the PlA1 alloantigen. Br J Haematol. 1982 Jun;51(2):297–304. [PubMed] [Google Scholar]
- Moeremans M., Daneels G., De Mey J. Sensitive colloidal metal (gold or silver) staining of protein blots on nitrocellulose membranes. Anal Biochem. 1985 Mar;145(2):315–321. doi: 10.1016/0003-2697(85)90368-9. [DOI] [PubMed] [Google Scholar]
- Nugent D. J., Kunicki T. J., Berglund C., Bernstein I. D. A human monoclonal autoantibody recognizes a neoantigen on glycoprotein IIIa expressed on stored and activated platelets. Blood. 1987 Jul;70(1):16–22. [PubMed] [Google Scholar]
- Nurden A. T., Didry D., Kieffer N., McEver R. P. Residual amounts of glycoproteins IIb and IIIa may be present in the platelets of most patients with Glanzmann's thrombasthenia. Blood. 1985 Apr;65(4):1021–1024. [PubMed] [Google Scholar]
- Pfueller S. L., Bilston R. A., Logan D., Gibson J. M., Firkin B. G. Heterogeneity of drug-dependent platelet antigens and their antibodies in quinine- and quinidine-induced thrombocytopenia: involvement of glycoproteins Ib, IIb, IIIa, and IX. Blood. 1988 Oct;72(4):1155–1162. [PubMed] [Google Scholar]
- Pfueller S. L., David R. Liberation of surface and internal platelet-associated IgG (PA-IgG) during platelet activation. Br J Haematol. 1986 Aug;63(4):785–794. doi: 10.1111/j.1365-2141.1986.tb07562.x. [DOI] [PubMed] [Google Scholar]
- Pfueller S. L., Firkin B. G., McGrath K. M., Logan D. Analysis of immunoglobulins that bind to platelets from serum of patients with immune thrombocytopenia: molecular weight distribution. Thromb Res. 1987 Aug 1;47(3):305–314. doi: 10.1016/0049-3848(87)90144-7. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puszkin E. G., Jenkins C. S., Ores-Carton C., Zucker M. B. Platelet cytoskeleton alpha-actinin in normal and thrombasthenic platelets: distribution and immunologic characterization. J Lab Clin Med. 1985 Jan;105(1):52–62. [PubMed] [Google Scholar]
- Steiner M., Lüscher E. F. Identification of the immunoglobulin G receptor of human platelets. J Biol Chem. 1986 Jun 5;261(16):7230–7235. [PubMed] [Google Scholar]
- Steiner M. Platelet-associated IgG is a specific protein. Biochem Biophys Res Commun. 1985 May 31;129(1):206–212. doi: 10.1016/0006-291x(85)91423-8. [DOI] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Murine natural monoclonal autoantibodies: a study of their polyspecificities and their affinities. Immunol Rev. 1986 Dec;94:99–112. doi: 10.1111/j.1600-065x.1986.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J. R., Pedersen J. S., Chalmers P. J., Toh B. H. Hybrids from normal, germ free, nude and neonatal mice produce monoclonal autoantibodies to eight different intracellular structures. Clin Exp Immunol. 1985 May;60(2):417–426. [PMC free article] [PubMed] [Google Scholar]
- von dem Borne A. E., Verheugt F. W., Oosterhof F., von Riesz E., de la Rivière A. B., Engelfriet C. P. A simple immunofluorescence test for the detection of platelet antibodies. Br J Haematol. 1978 Jun;39(2):195–207. doi: 10.1111/j.1365-2141.1978.tb01089.x. [DOI] [PubMed] [Google Scholar]