Abstract
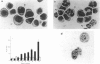
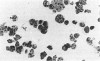
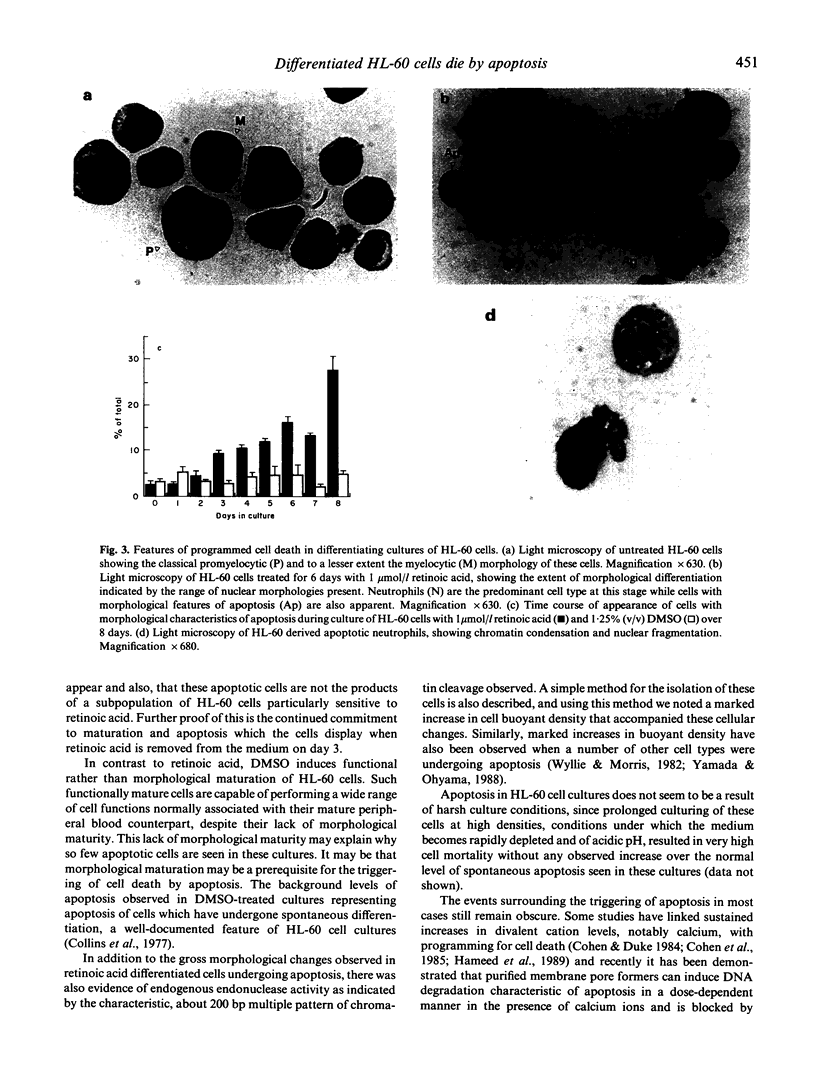
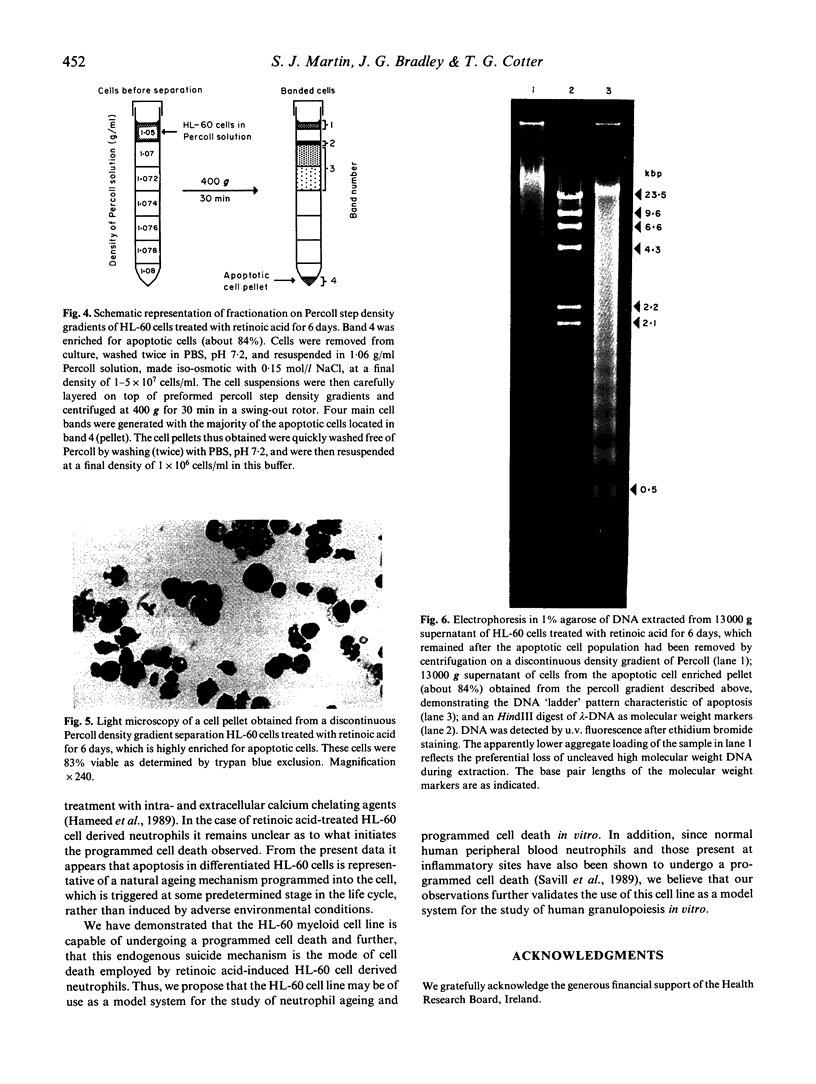
The human promyelocytic HL-60 cell line can be induced to differentiate to neutrophil-like cells in response to a variety of chemical stimuli. We have found that retinoic acid-treatment of HL-60 cells over a period of 6-8 days resulted in a progressive increase in the proportion of cells with mature neutrophil morphologies and was closely followed by an increase in the proportion of cells exhibiting the morphological characteristics of apoptosis, the non-pathological mode of cell death. Using Percoll step-density gradients we have demonstrated a marked increase in the buoyant density of these cells and have used this density difference to obtain enriched fractions of cells for more detailed study. Degradation of the nuclear DNA of these cells into integer multiples of about 200 base pairs, indicative of endogenous endonuclease activation a major characteristic of programmed cell death, was also demonstrated. From these observations we conclude that the mode of cell death in cultures of terminally differentiated HL-60 cells is that of apoptosis. These results parallel those of a recent report which has shown apoptosis to be the mode of cell death of ageing peripheral blood neutrophils. Because of this, we believe that our observations further validate the use of the HL-60 cell line as a model system for the study of human granulopoiesis in vitro and further, that this model system may be useful for gaining insight into the underlying mechanisms involved in apoptosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitman T. R., Collins S. J., Keene B. R. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 1981 Jun;57(6):1000–1004. [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplinski T. J., Sloan G. J., Niedel J. E. Granulocyte functions during maturation of human promyelocytic leukemia cells. Leuk Res. 1985;9(7):897–903. doi: 10.1016/0145-2126(85)90311-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Chervenak R., Sellins K. S., Olson L. K. DNA fragmentation in targets of CTL: an example of programmed cell death in the immune system. Adv Exp Med Biol. 1985;184:493–508. doi: 10.1007/978-1-4684-8326-0_32. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Hameed A., Olsen K. J., Lee M. K., Lichtenheld M. G., Podack E. R. Cytolysis by Ca-permeable transmembrane channels. Pore formation causes extensive DNA degradation and cell lysis. J Exp Med. 1989 Mar 1;169(3):765–777. doi: 10.1084/jem.169.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Greenberger J. S., Cohen H. J. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979 Aug;82(2):315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G. Hormone-induced cell death. Purification ad properties of thymocytes undergoing apoptosis after glucocorticoid treatment. Am J Pathol. 1982 Oct;109(1):78–87. [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Ohyama H. Radiation-induced interphase death of rat thymocytes is internally programmed (apoptosis). Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Jan;53(1):65–75. doi: 10.1080/09553008814550431. [DOI] [PubMed] [Google Scholar]
- von Melchner H., Höffken K. Commitment to differentiation of human promyelocytic leukemia cells (HL60): an all-or-none event preceded by reversible losses of self-renewal potential. J Cell Physiol. 1985 Dec;125(3):573–581. doi: 10.1002/jcp.1041250329. [DOI] [PubMed] [Google Scholar]