Abstract
Delayed-type hypersensitivity (DTH) reactions are antigen-specific cell-mediated immune responses that, depending on the antigen, mediate beneficial (e.g., resistance to viruses, bacteria, and fungi) or harmful (e.g., allergic dermatitis and autoimmunity) aspects of immune function. Contrary to the idea that stress suppresses immunity, we have reported that short-duration stressors significantly enhance skin DTH and that a stress-induced trafficking of leukocytes to the skin may mediate this immunoenhancement. Here, we identify the hormonal mediators of a stress-induced enhancement of skin immunity. Adrenalectomy, which eliminates the glucocorticoid and epinephrine stress response, eliminated the stress-induced enhancement of skin DTH. Low-dose corticosterone or epinephrine administration significantly enhanced skin DTH and produced a significant increase in the number of T cells in lymph nodes draining the site of the DTH reaction. In contrast, high-dose corticosterone, chronic corticosterone, or low-dose dexamethasone administration significantly suppressed skin DTH. These results suggest a role for adrenal stress hormones as endogenous immunoenhancing agents. These results also show that hormones released during an acute stress response may help prepare the immune system for potential challenges (e.g., wounding or infection) for which stress perception by the brain may serve as an early warning signal.
Stress, a familiar aspect of modern life, is a stimulant for some but a concern for many. We have defined stress as a constellation of events, beginning with a stimulus (stressor) that precipitates a reaction in the brain (stress perception) that subsequently activates physiologic systems in the body (stress response) (1). Stress has long been suspected of playing a role in the etiology of many diseases, and numerous studies have shown that stress can be immunosuppressive and hence may be detrimental to health (2–10). Moreover, glucocorticoid stress hormones are regarded widely as being immunosuppressive (2) and are used clinically as antiinflammatory agents (11).
However, suppression of immune function under all stress conditions would not be evolutionarily adaptive. Stress is an intrinsic part of life for most organisms, and dealing successfully with stressors enables survival. Environmental challenges and most evolutionary selection pressures are stressors that may be psychological (fear or anxiety), physical (injury or infection), or physiological (deprivation of food or water). One of the primary functions of the brain is to perceive stress and warn and enable an organism to deal with its consequences. For example, when a gazelle sees a charging lion, the gazelle’s brain detects a threat and orchestrates a physiologic response that first prepares and then enables the gazelle to flee. We have suggested that such stress conditions may also prepare the immune system for challenges (e.g., wounding or infection) that may be imposed by the stressor, just as stress conditions prepare the nervous, cardiovascular, musculoskeletal, and neuroendocrine systems for fight or flight (1, 12–15). A focus of our research has been to elucidate the cellular and molecular mechanisms mediating the beneficial versus harmful effects of stress on the overall health of an organism.
We hypothesized that stress may have bidirectional effects on immune function such that acute stress may be immunoenhancing, whereas chronic stress may be immunosuppressive (1, 16). Initial studies in rats showed that acute stress (2-h restraint) results in a significant redistribution of leukocytes from the blood to other organs (skin, lymph nodes, bone marrow) in the body (12, 13, 17), and that adrenal stress hormones are the major mediators of this leukocyte redistribution (14). Because the skin was one of the targets to which leukocytes trafficked during stress, we hypothesized that such a leukocyte redistribution may increase immune surveillance and consequently enhance immune function should the skin be exposed to antigen after acute stress. To test this hypothesis, we examined the effects of acute stress on skin immunity by using a model for delayed-type hypersensitivity (DTH) that is an example of antigen-specific cell-mediated immunity. Acute stress administered immediately before the introduction of an antigenic challenge resulted in a large and long-lasting enhancement of skin DTH (15). Histological analysis identified significantly larger numbers of leukocytes in the skin of stressed animals both before and after exposure to antigen, suggesting that a stress-induced redistribution of leukocytes was one of the factors mediating the stress-induced enhancement of skin immunity (15). In contrast to the immunoenhancing effects of acute stress, we also showed that chronic stress significantly suppressed the DTH response (1). The experiments described here elucidate the role of the adrenal hormones corticosterone and epinephrine as mediators of the bidirectional effects of stress on skin immunity.
MATERIALS AND METHODS
Animals.
Young adult male Sprague–Dawley rats (150–300 g; Harlan–Sprague–Dawley) were housed in plastic cages in the accredited (American Association for the Accreditation of Laboratory Animal Care) animal facilities of The Rockefeller University. The animal room was maintained on a 12-h light-dark cycle (lights on at 7 a.m.). Animals were given rat chow and water ad libitum.
Adrenalectomy (ADX).
Bilateral ADX was performed with standard aseptic surgical techniques on animals fully anesthetized with the inhalant methoxyflurane (Metofane; Pitman–Moore, Washington Crossing, NJ). Sham-adrenalectomized (sham) animals went through exactly the same procedure as ADX animals, except that their adrenal glands were not removed. ADX animals were maintained on a low “normalizing” dose of corticosterone (20 μg/ml) administered through drinking fluid (animals were given two bottles, one with water and one with 3% saline). This maintenance was necessary for restoring important permissive functions of corticosterone that are lost after ADX (18–20). Corticosterone replacement normalizes (data not shown) basal levels of adrenocorticotropic hormone, blood-leukocyte numbers, and catecholamine hormones (21), all of which are abnormally high in ADX animals. Unlike constant replacement (via pellets or osmotic pumps), drinking-water corticosterone facilitates the normal termination of a stress-induced adrenocorticotropic hormone response (22, 23). Drinking-water corticosterone also simulates the circadian corticosterone rhythm, because animals drink at the beginning of the active period (16).
Stress.
Acute stress was administered by placing animals (without squeezing or compression) in well ventilated Plexiglas restrainers for 2 h. This procedure approximates stress that is largely psychological in nature because of the perception of confinement on part of the animal (for review see refs. 24 and 25). Restraint activates the autonomic nervous system (21) and the hypothalamic–pituitary–adrenal axis (26–28) and results in the activation of adrenal steroid receptors throughout the body (26, 27).
Hormone Administration.
The concentration and timing of each hormone administered is listed below and in the figures. Vehicle or hormone was administered via i.p. injection. Corticosterone and dexamethasone (Sigma) were dissolved in an aqueous solution of 30% 2-hydroxypropyl-β-cyclodextrin (HBC; Research Biochemicals, Natick, MA). Epinephrine (Research Biochemicals) was dissolved in sterile water.
Induction of DTH.
DTH was induced by challenging the pinnae of previously sensitized rats with 2,4-dinitro-1-fluorobenzene (DNFB; Sigma) or oxazolone (OXA; Sigma). On day 1 of sensitization, animals were anesthetized with methoxyflurane. No anesthesia was used subsequently. An area of approximately 3 × 4 cm was shaved on the dorsum. The thickness of both pinnae was recorded by using a constant-loading dial micrometer (Mitutoyo, Tokyo, Japan). On days 1 and 2 of sensitization, 100 μl of DNFB [1% (wt/vol) in 4:1, acetone:olive oil] or 100 μl of OXA [1.5% (wt/vol) in ethanol] was applied to the shaved dorsum. On day 5, baseline pinna thickness was measured. On day 6, after stress or hormone administration, the dorsal surface of the right pinnae of all animals was challenged with 50 μl of DNFB [0.5% (wt/vol) in 4:1 acetone:olive oil] or OXA [0.75% (wt/vol) in ethanol]. Left pinnae were treated with vehicle. Pinna thickness was measured at the times shown with all measurements being made on same relative region of the pinna. Vehicle-treated (left) pinnae showed no significant change in thickness (data not shown).
The immune reaction induced by using the above procedure is characterized by swelling at the site of challenge and by an infiltration of monocytes/macrophages and lymphocytes into the epidermis and dermis (29–31). A positive correlation between the intensity of the immune reaction and the increase in pinna thickness has been reported (32, 33). This model for skin DTH reactions has been used widely to monitor cell-mediated immune responses in vivo (30, 34).
Isolation of Lymphocytes from Cervical Lymph Nodes.
Cervical lymph nodes were dissected and placed in sterile PBS on ice (n = 3 per treatment group). Subsequently, each lymph node was weighed and disrupted between the frosted ends of two microscope slides. Suspensions of leukocytes were prepared in PBS and stored on ice for cell counting, immunofluorescent staining, and flow cytometry.
Flow Cytometric Analysis of Leukocytes.
White blood cell counts and lymphocyte-neutrophil differentials were obtained on a hematology analyzer (F800, Sysmex, McGraw Park, IL). Specific leukocyte subtypes were measured by immunofluorescent antibody staining and subsequent analysis by using three-color flow cytometry (FACScan, Becton Dickinson). T cells were identified by using the following mAbs from Caltag (South San Francisco, CA): CD3-FITC (1F4), CD4-PE (w3/25), and CD8-TC (OX8). Briefly, cell suspensions were incubated with antibody for 20 min at room temperature, washed with PBS, and read on the FACScan with 3,000–5,000 events being acquired from each preparation. Appropriate isotype controls were used to set the negative criteria. Data were analyzed with cellquest software (Becton Dickinson).
Data Analysis and Statistics.
For all experiments, repeated measures were made for each animal. Differences between time points were analyzed with Student’s t test as a test for significant differences between means. Means that differed significantly are indicated by symbols that are defined in the figure legends. Data are expressed as mean ± SEM. A computer statistics package was used for statistical analyses (systat, version 5.2.1, Systat, Evanston, IL).
RESULTS
Adrenal Hormones Mediate the Stress-Induced Enhancement of Skin DTH.
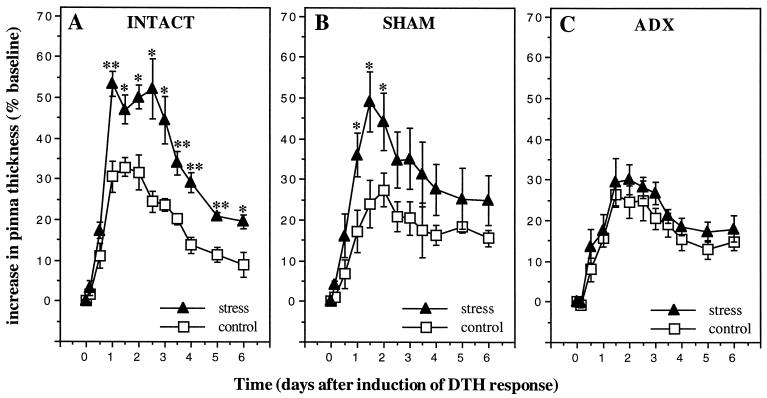
In the tradition of classical endocrinology, we hypothesized that if the stress-induced enhancement of skin DTH were mediated by adrenal hormones, ADX would reduce or eliminate the immunoenhancing effects of acute stress. We compared the effects of stress on the DTH response of adrenal-intact (intact), sham, and ADX animals (Fig. 1). DTH responses of two sets of animals were examined within each treatment group. One group of intact, sham, and ADX animals (n = 6 per group) was undisturbed before antigen administration (control group). Another group of intact, sham, and ADX animals was restrained for 2 h immediately before antigen administration (stress group). Fig. 1 shows that intact and sham animals showed a significant stress-induced enhancement of skin DTH, whereas ADX animals did not.
Figure 1.
Adrenalectomy eliminates a stress-induced enhancement of skin DTH. A 6-day time course of changes in thickness of right pinnae of previously sensitized animals challenged with DNFB is shown. Stressed intact (A) and sham (B) animals showed a significant increase in the DTH response compared with unstressed animals. (C) ADX animals did not show a stress-induced increase skin DTH. Data are expressed as means ± SEM (n = 6 per treatment group). Statistically significant differences are indicated. (∗, P < 0.05; ∗∗, P < 0.005, independent t test.)
Immunoenhancing Effects of Low Doses of Corticosterone on Skin DTH.
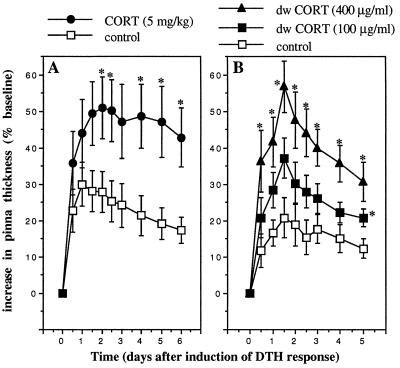
The experiments described above showed that adrenal hormones released during stress were the major mediators of the stress-induced enhancement of skin DTH. However, the adrenal gland is the source of two principal stress hormones, the glucocorticoid, corticosterone, and the catecholamine, epinephrine. It was important to elucidate the role of each of these hormones in mediating the immunoenhancing effects of stress. Fig. 2A shows the DTH response of ADX animals challenged with DNFB 2 h after the administration of saline (control, n = 5) or of a low dose of corticosterone (5 mg/kg, n = 5). Without adrenal glands, ADX animals were incapable of mounting a corticosterone stress response. Fig. 2A shows that acute administration of a low dose of corticosterone to ADX animals, which mimicked the corticosterone response of intact animals, induced a significant enhancement of skin DTH.
Figure 2.
Acute administration of corticosterone enhances skin DTH. A time course of changes in the thickness of right pinnae of previously sensitized animals challenged with DNFB is shown. Corticosterone (CORT, 5 mg/kg) was administered i.p. to ADX animals (A) or through drinking water (100 or 400 μg/ml) to intact animals (B). Control animals were treated with vehicle: 30% HBC (A) or 0.6% ethanol (B). Corticosterone-treated animals showed a significantly larger DTH response than vehicle-treated animals. (∗, P < 0.05, independent t test.)
To validate the finding that corticosterone, generally regarded as an immunosuppressive hormone, can enhance cell-mediated immunity in vivo, we tested the effects of acutely administering the hormone further by using a noninvasive technique and intact animals (Fig. 2B). Vehicle (0.6% ethanol) or corticosterone (100 μg/ml or 400 μg/ml) was administered to different groups (n = 5) of animals in drinking water for a period of 4 h, starting 2 h before and ending 2 h after the beginning of the active period of the diurnal cycle when all animals were challenged with DNFB (n = 5). Because animals typically start drinking at the beginning of their active period, the animals self-administered corticosterone, after which they were challenged with antigen. The plasma levels of corticosterone attained were similar to those observed during stress. Fig. 2B shows that this noninvasive corticosterone administration also resulted in a significant enhancement of skin DTH.
Immunosuppressive Effects of Glucocorticoid Hormones on Skin DTH.
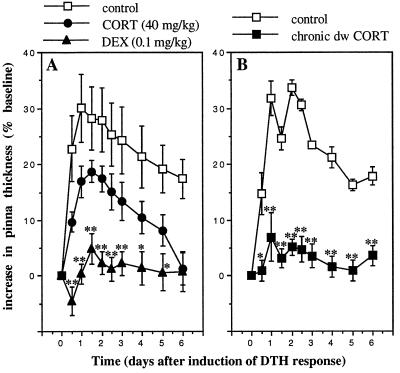
The studies described in Fig. 2 examine the effects of physiologic levels of corticosterone, and the studies described in Fig. 3 examine the effects of pharmacologic treatments with glucocorticoid hormones on skin DTH. Acute administration of a high dose of corticosterone (40 mg/kg), the endogenous glucocorticoid hormone, or a low dose of dexamethasone (0.1 mg/kg), a synthetic glucocorticoid, to ADX animals significantly suppressed skin DTH (Fig. 3A; n = 5). Similar results were observed after corticosterone or dexamethasone administration to intact animals (data not shown). Moreover, chronic (6-day) administration of drinking-water corticosterone (400 μg/ml; n = 5) significantly suppressed skin DTH (Fig. 3B). Notably, this result was in contrast to acute (4-h) administration of the same dose of drinking-water corticosterone, which enhanced the DTH response (Fig. 2B).
Figure 3.
Pharmacologic treatment with glucocorticoid hormones suppresses the skin DTH response. A time course of changes in the thickness of right pinnae of previously sensitized animals challenged with DNFB is shown. (A) Corticosterone (CORT, 40 mg/kg) or dexamethasone (DEX, 0.1 mg/kg) was administered acutely. (B) Corticosterone was also administered chronically in drinking water (dw CORT, 400 μg/ml, 6 days). Control animals were treated with vehicle: 30% HBC (A) or 0.6% ethanol (B). Corticosterone- and dexamethasone-treated animals showed lower DTH responses than control animals. (∗, P < 0.05; ∗∗, P < 0.005, independent t test.)
Immunoenhancing Effects of Epinephrine on Skin DTH.
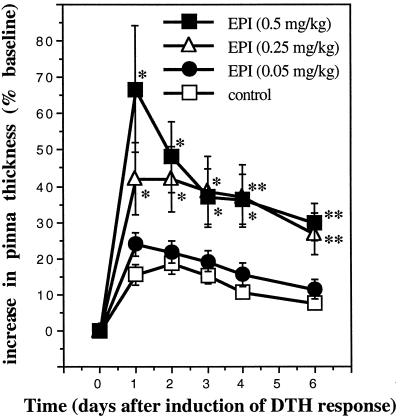
Fig. 4 shows the DTH response of ADX animals challenged with DNFB 2 h after the administration of water (vehicle) or increasing doses (0.05, 0.25, 0.5 mg/kg) of epinephrine (n = 6). Compared with vehicle-treated controls, animals treated acutely with epinephrine showed a significant dose-dependent enhancement of skin DTH.
Figure 4.
Acute administration of epinephrine enhances skin DTH. A time course of changes in the thickness of right pinnae of previously sensitized animals challenged with DNFB is shown. Epinephrine (EPI, 0.05, 0.25, or 0.5 mg/kg) was administered acutely to ADX animals. Control animals were treated with vehicle (deionized-distilled H2O). Epinephrine-treated animals showed a dose-dependent increase in skin DTH. (∗, P < 0.05; ∗∗, P < 0.005, independent t test.)
Corticosterone and Epinephrine Produce an Additive Enhancement of Skin DTH.
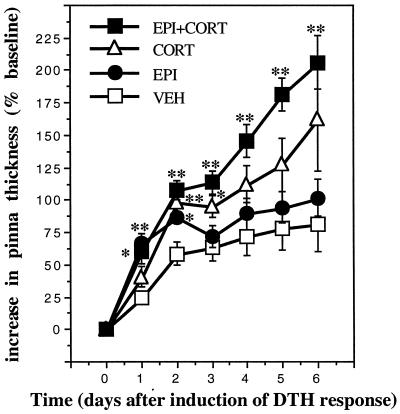
The experiments described above showed that manipulations designed to mimic acute stress-induced changes in either corticosterone or epinephrine enhanced skin DTH. However, a physiologic stress response consists of increased plasma levels of both corticosterone and epinephrine. Therefore, we examined potential interactions between corticosterone and epinephrine with respect to skin DTH. To test the applicability of our findings to an antigen other than DNFB, OXA was used in this experiment. ADX animals were injected with vehicle (30% HBC), epinephrine (500 μg/kg), corticosterone (5 mg/kg), or epinephrine plus corticosterone (500 μg/kg plus 5 mg/kg, respectively) (Fig. 5; n = 6). Animals were challenged with OXA 2 h after hormone administration. Epinephrine or corticosterone administration enhanced skin DTH, but corticosterone induced a greater immunoenhancement than epinephrine. Moreover, simultaneous administration of epinephrine and corticosterone produced an even greater increase in the DTH response. These results suggest that epinephrine and corticosterone may act additively to enhance skin DTH.
Figure 5.
Epinephrine and corticosterone additively enhance skin DTH. A time course of changes in the thickness of right pinnae of previously sensitized animals challenged with OXA is shown. Epinephrine (EPI, 0.5 mg/kg), corticosterone (CORT, 5 mg/kg) or epinephrine plus corticosterone (EPI+CORT, 0.5 mg/kg plus 5 mg/kg, respectively) were administered acutely to ADX animals. Control animals were treated with vehicle (VEH, 30% HBC). Epinephrine- or corticosterone-treated animals showed an enhanced DTH response. Moreover, simultaneous administration of the two hormones resulted in an additive enhancement of skin DTH. (∗, P < 0.05; ∗∗, P < 0.005, independent t test.)
Stress Hormones Increase the Number of T Cells in Lymph Nodes That Drain a Skin DTH Response.
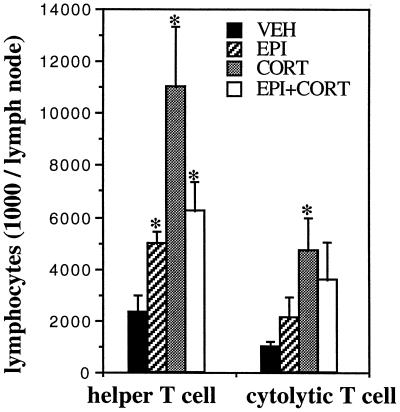
We have shown previously that stress-induced trafficking of leukocytes from the blood to the skin may be one of the mechanisms by which acute stress enhances a skin DTH response (15). In the present series of studies, we examined the effects of different hormone treatments on the cellularity of cervical lymph nodes that drain the site (ear) of the DTH response. ADX animals were injected with vehicle (30% HBC), epinephrine (500 μg/kg), corticosterone (5 mg/kg), or epinephrine in conjunction with corticosterone (500 μg/kg and 5 mg/kg, respectively) (n = 3). All animals were challenged with OXA 2 h after hormone administration. Absolute numbers of helper T cells (CD3+ CD4+) and cytolytic T cells (CD3+ CD8+) were measured 48 h after antigen exposure. Fig. 6 shows that compared with vehicle-treated animals, hormone-treated animals showed significantly higher numbers of lymphocytes in cervical lymph nodes.
Figure 6.
Acute administration of stress hormones increases the cellularity of cervical lymph nodes that drain the site of the skin DTH reaction. Epinephrine (EPI, 0.5 mg/kg), corticosterone (CORT, 5 mg/kg), or epinephrine plus corticosterone (EPI+CORT, 0.5 mg/kg plus 5 mg/kg, respectively) were administered to ADX animals. Control animals were treated with vehicle (VEH, 30% HBC). Lymph nodes were collected, and lymphocytes were isolated 48 h after the induction of DTH. Compared with vehicle-treated animals, hormone-treated animals showed higher T lymphocyte numbers in cervical lymph nodes that drain the site of the DTH reaction. (∗, P < 0.05, independent t test.)
DISCUSSION
Stress and stress hormones have long been regarded as being immunosuppressive (2–7). However, suppression of immune function under all stress conditions would be evolutionarily maladaptive. It seems paradoxical that organisms should have evolved to suppress immune function at a time when an active immune response may be critical for survival, for example, under conditions of stress when an organism may be injured or infected by the actions of the stress-inducing agent (e.g., a predator). Another paradoxical observation is that, on the one hand, stress is thought to suppress immunity and increase susceptibility to infections and cancer (8, 10, 35–37). On the other, it is thought to exacerbate inflammatory diseases (38–40), like psoriasis, asthma, and arthritis, which should be ameliorated by a suppression of immune function.
Keeping these considerations in mind and based on our initial observations on the effects of stress and of the circadian corticosterone rhythm on leukocyte redistribution in the body (12, 13), we showed that stress has bidirectional effects on immune function, such that acute stress is immunoenhancing, whereas chronic stress is immunosuppressive (1, 13, 15). The studies described in the present paper show that hormonal manipulations that mimic acute stress produce enhancing effects on skin immunity, whereas those that mimic chronic stress suppress skin immunity.
These findings may help to explain the paradoxical situations described above. For example, under natural conditions, acute stress may serve a protective role by enhancing an immune response directed toward a wound or infection. However, a stress-induced enhancement of immune function could also be detrimental if the immune response were directed against an innocuous (e.g., poison ivy, nickel in jewelry, or latex) or autoimmunogenic antigen. This hypothesis could explain the well known stress-induced exacerbation of autoimmune diseases (38–40). Moreover, we have shown that chronic stress (1) and hormonal conditions that mimic chronic stress suppress immune function. This fact may explain stress-induced exacerbation of infections and cancer (10, 35–37) and stress-induced suppression of wound healing (9, 41). We have also shown here that high-dose or prolonged administration of corticosterone or acute administration of low doses of dexamethasone are potently immunosuppressive, consistent with their clinically well known antiinflammatory effects.
These studies underline the importance of distinguishing between physiologic versus pharmacologic concentration and kinetic parameters when examining the effects of stress hormones on immune function. Thus, low doses and acute administration of corticosterone and epinephrine, which mimic acute stress, produce immunoenhancement. Increasing the concentration of corticosterone to pharmacologic levels or increasing the duration of corticosterone exposure to mimic levels observed during chronic stress produces immunosuppression. Importantly, dexamethasone, a widely used synthetic analog of corticosterone, is potently immunosuppressive, possibly because dexamethasone bypasses several physiologic buffering mechanisms that restrict corticosterone from accessing tissues in vivo. First, dexamethasone does not bind corticosteroid-binding globulin, a plasma protein that binds a large proportion of circulating corticosterone (42) and hence prevents it from activating glucocorticoid receptors in certain tissues (26, 42, 43). Second, dexamethasone has a significantly longer half-life than corticosterone (44, 45). Third, dexamethasone has a higher affinity for glucocorticoid receptors (46) and is significantly more efficient than corticosterone at activating glucocorticoid receptors in vivo (43).
Our data are consistent with those of other studies showing bidirectional effects of corticosterone on T cell proliferation (47) and stress-induced enhancements in in vitro parameters such as lymphocyte proliferation (48–51), macrophage phagocytosis (52), natural killer cell activity (53, 54), and cytokine production (55, 56). Acute stress has also been shown to enhance skin DTH (57), accelerate antigen removal (58), and increase antigen-specific antibody titers in vivo (59–62).
In light of our findings (1, 12–15), we have proposed a model in which stress hormones, cell-adhesion molecules, cytokines, and chemokines may act in concert to promote an acute stress-induced enhancement of skin immunity (1, 17). According to this model, stress hormones induce an increase in the affinity/expression of adhesion molecules on leukocytes and/or endothelial cells in compartments such as the skin and lymph nodes. This increase in endothelial “adhesivity” results in a selective retention of leukocytes within these compartments and increases immune surveillance. If the stress signal is followed by inflammatory mediator signals (released because of wounding or infection) at the site of leukocyte margination, leukocytes transmigrate through the endothelial lining and infiltrate the site of inflammation. Thus, a stressed organism may mount a more vigorous immune response by virtue of having more leukocytes at a site of challenge compared with a nonstressed animal.
In this manner, stress hormones may direct the body’s “soldiers” (leukocytes), to exit their “barracks” (spleen and bone marrow), travel the “boulevards” (blood vessels), and take position at potential “battle stations” (skin, lining of gastrointestinal and urinary-genital tracts, and draining lymph nodes) (1, 12–15). Moreover, we hypothesize that, in addition to sending leukocytes to potential battle stations, stress hormones may also better equip them for battle by enhancing processes like antigen presentation, phagocytosis, cytokine function, and antibody production (1). Thus, a hormonal alarm signal released by the brain on detecting a stressor may prepare the immune system for potential challenges (wounding or infection) that may arise from the actions of the stress-inducing agent (e.g., a predator or attacker). In contrast, it is likely that chronic stress suppresses immune function by decreasing leukocyte redistribution (1) and by inhibiting cytokine and prostaglandin synthesis and leukocyte function (11, 63).
The studies described here are important, because stress is suspected to play a role in the etiology of many diseases. Moreover, glucocorticoid as well as catecholamine hormones are prescribed for numerous clinical conditions (64, 65). A determination of the physiologic mechanisms through which stress and stress hormones enhance or suppress immune responses may help our understanding and treatment of some of these diseases. Thus, future studies will aim to facilitate the development of biomedical treatments designed to harness an individual’s physiology to enhance (during vaccination, wounding, infections, or cancer) or suppress (during autoimmune or inflammatory disorders) the immune response selectively, depending on what would be most beneficial for the patient.
Acknowledgments
We thank Anisha Patel and Michelle Wyland for assistance with hormone administration. This research was supported by The John D. and Catherine T. MacArthur Foundation.
ABBREVIATIONS
- ADX
adrenalectomy
- DNFB
2,4-dinitro-1-fluorobenzene
- DTH
delayed-type hypersensitivity
- HBC
2-hydroxypropyl-β-cyclodextrin
- OXA
oxazolone
References
- 1.Dhabhar F S, McEwen B S. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 2.Munck A, Guyre P M, Holbrook N J. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 3.Borysenko M, Borysenko J. Gen Hosp Psychiatry. 1982;4:59–67. doi: 10.1016/0163-8343(82)90028-7. [DOI] [PubMed] [Google Scholar]
- 4.Khansari D N, Murgo A J, Faith R E. Immunol Today. 1990;11:170–175. doi: 10.1016/0167-5699(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 5.Kort W J. Adv Neuroimmunol. 1994;4:1–11. doi: 10.1016/s0960-5428(06)80186-5. [DOI] [PubMed] [Google Scholar]
- 6.Maier S F, Watkins L R, Fleshner M. Am Psychol. 1994;49:1004–1017. doi: 10.1037//0003-066x.49.12.1004. [DOI] [PubMed] [Google Scholar]
- 7.Herbert T B, Cohen S. Psychosom Med. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser J K, Glaser R, Gravenstein S, Malarkey W B, Sheridan J. Proc Natl Acad Sci USA. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marucha P T, Kiecolt-Glaser J K, Favagehi M. Psychosom Med. 1998;60:362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan J F. Brain Behav Immun. 1998;12:1–6. doi: 10.1006/brbi.1998.0521. [DOI] [PubMed] [Google Scholar]
- 11.Schleimer R P, Claman H N, Oronsky A. Anti-Inflammatory Steroid Action. San Diego: Academic; 1989. [Google Scholar]
- 12.Dhabhar F S, Miller A H, Stein M, McEwen B S, Spencer R L. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- 13.Dhabhar F S, Miller A H, McEwen B S, Spencer R L. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- 14.Dhabhar F S, Miller A H, McEwen B S, Spencer R L. J Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- 15.Dhabhar F S, McEwen B S. J Immunol. 1996;156:2608–2615. [PubMed] [Google Scholar]
- 16.Dhabhar F S. Dissertation. New York: Rockefeller Univ.; 1996. [Google Scholar]
- 17.Dhabhar F S. Ann NY Acad Sci. 1998;840:359–372. doi: 10.1111/j.1749-6632.1998.tb09575.x. [DOI] [PubMed] [Google Scholar]
- 18.Ingle D J. Acta Endocrinol. 1954;17:172–186. [PubMed] [Google Scholar]
- 19.Ambrose C T. J Exp Med. 1964;119:1027–1049. doi: 10.1084/jem.119.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Kloet E R. Front Neuroendocrinol. 1995;16:416–425. doi: 10.1006/frne.1995.1015. [DOI] [PubMed] [Google Scholar]
- 21.Kvetnansky R, Fukuhara K, Pacak K, Cizza G, Goldstein D S, Kopin I J. Endocrinology. 1993;133:1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- 22.Akana S F, Jacobson L, Cascio C S, Shinsako J, Dallman M F. Endocrinology. 1988;122:1337–1342. doi: 10.1210/endo-122-4-1337. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson L, Akana S F, Cascio C S, Shinsako J, Dallman M F. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- 24.Berkenbosch F, Wolvers D A, Derijk R. J Steroid Biochem Mol Biol. 1991;40:639–647. doi: 10.1016/0960-0760(91)90286-e. [DOI] [PubMed] [Google Scholar]
- 25.Glavin G B, Paré W P, Sandbak T, Bakke H-K, Murison R. Neurosci Biobehav Rev. 1994;18:223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 26.Dhabhar F S, Miller A H, McEwen B S, Spencer R L. J Neuroimmunol. 1995;56:77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- 27.Plotsky P M, Meaney M J. Brain Res Molec Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 28.Dhabhar F S, McEwen B S, Spencer R L. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- 29.Vadas M A, Miller J F A P, Gamble J, Whitelaw A. Int Arch Allergy Appl Immunol. 1975;49:670–692. doi: 10.1159/000231449. [DOI] [PubMed] [Google Scholar]
- 30.Turk J L. Delayed Hypersensitivity Research Monographs in Immunology. Amsterdam: Elsevier; 1980. [Google Scholar]
- 31.Malorny U, Goebeler M, Gutwald J, Roth J, Sorg C. Int Arch Allergy Appl Immunol. 1990;92:356–360. doi: 10.1159/000235164. [DOI] [PubMed] [Google Scholar]
- 32.Phanuphak P, Moorhead J W, Claman H N. J Immunol. 1974;112:115–123. [PubMed] [Google Scholar]
- 33.Kimber I, Dearman R. J Pharmacol Toxicol Methods. 1993;29:11–16. doi: 10.1016/1056-8719(93)90045-g. [DOI] [PubMed] [Google Scholar]
- 34.Thorne P S, Hawk C, Kaliszweski S D, Guiney P D. Fundam Appl Toxicol. 1991;17:790–806. doi: 10.1016/0272-0590(91)90186-8. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Eliyahu S, Yirmiya R, Liebeskind J C, Taylor A N, Gale R P. Brain Behav Immun. 1991;5:193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Tyrrell D A J, Smith A P. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 37.Glaser R, Pearl D K, Kiecolt-Glaser J K, Malarkey W B. Psychoneuroendocrinology. 1994;19:765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 38.Solomon G F, Moos R H. Arch Gen Psychiat. 1964;11:657–669. doi: 10.1001/archpsyc.1964.01720300087011. [DOI] [PubMed] [Google Scholar]
- 39.Mei-Tal V, Meyerowitz S, Engel G. Psychosom Med. 1970;32:67–86. doi: 10.1097/00006842-197001000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Thomason B T, Brantley P J, Jones G N, Dyer H R, Morris J L. J Behav Med. 1992;15:215–220. doi: 10.1007/BF00848326. [DOI] [PubMed] [Google Scholar]
- 41.Padgett D A, Marucha P T, Sheridan J F. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 42.Siiteri P K, Murai J T, Hammond G L, Nisker J A, Raymoure W J, Kuhn R W. Recent Prog Horm Res. 1982;38:457–503. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- 43.Spencer R L, Young E A, Choo P H, McEwen B S. Brain Res. 1990;514:37–48. doi: 10.1016/0006-8993(90)90433-c. [DOI] [PubMed] [Google Scholar]
- 44.Siiteri P K, Simberg N H. Clin Endocrinol Metab. 1986;15:247–258. doi: 10.1016/s0300-595x(86)80023-8. [DOI] [PubMed] [Google Scholar]
- 45.Monder C, Miroff Y, Marandici A, Hardy M P. Endocrinology. 1994;134:1199–1204. doi: 10.1210/endo.134.3.8119160. [DOI] [PubMed] [Google Scholar]
- 46.Svec F. J Steroid Biochem. 1985;23:669–671. doi: 10.1016/0022-4731(85)90020-2. [DOI] [PubMed] [Google Scholar]
- 47.Wiegers J G, Reul J M H M, Holsboer F, De Kloet E R. Endocrinology. 1994;135:2351–2357. doi: 10.1210/endo.135.6.7988417. [DOI] [PubMed] [Google Scholar]
- 48.Wood P G, Karol M H, Kusnecov A W, Rabin B S. Brain Behav Immun. 1993;7:121–134. doi: 10.1006/brbi.1993.1014. [DOI] [PubMed] [Google Scholar]
- 49.Rinner I, Schauenstein K, Mangge H, Porta S, Kvetnansky R. Brain Behav Immun. 1992;6:130–140. doi: 10.1016/0889-1591(92)90013-e. [DOI] [PubMed] [Google Scholar]
- 50.Lysle D T, Cunnick J E, Rabin B S. Brain Behav Immun. 1990;4:269–277. doi: 10.1016/0889-1591(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 51.Shurin M R, Zhou D, Kusnecov A, Rassnick S, Rabin B S. Brain Behav Immun. 1994;8:57–65. doi: 10.1006/brbi.1994.1005. [DOI] [PubMed] [Google Scholar]
- 52.Lyte M, Nelson S G, Thompson M L. Clin Immunol Immunopathol. 1990;57:137–147. doi: 10.1016/0090-1229(90)90029-p. [DOI] [PubMed] [Google Scholar]
- 53.Jain S, Stevenson J R. Immunol Invest. 1991;20:365–376. doi: 10.3109/08820139109057762. [DOI] [PubMed] [Google Scholar]
- 54.Millar D B, Thomas J R, Pacheco N D, Rollwagen F M. Brain Behav Immun. 1993;7:144–153. doi: 10.1006/brbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 55.Mekaouche M, Givalois L, Barbanel G, Siaud P, Maurel D, Malaval F, Bristow A F, Boissin J, Assenmacher I, Ixart G. Neuroimmunomodulation. 1994;1:292–299. doi: 10.1159/000097179. [DOI] [PubMed] [Google Scholar]
- 56.Petitto J M, Lysle D T, Gariepy J-L, Lewis M H. Brain Behav Immun. 1994;8:111–122. doi: 10.1006/brbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- 57.Blecha F, Barry R A, Kelley K W. Proc Soc Exp Biol Med. 1982;169:239–246. doi: 10.3181/00379727-169-41338. [DOI] [PubMed] [Google Scholar]
- 58.Sabiston B H, Rose J E, Cinader B. J Immuongenet. 1978;5:197–212. doi: 10.1111/j.1744-313x.1978.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 59.Solomon G F. Int Arch Allergy. 1969;35:97–104. doi: 10.1159/000230163. [DOI] [PubMed] [Google Scholar]
- 60.Blecha F, Kelley K W. J Anim Sci. 1981;53:439–447. doi: 10.2527/jas1981.532439x. [DOI] [PubMed] [Google Scholar]
- 61.Cocke R, Moynihan J A, Cohen N, Grota L J, Ader R. Brain Behav Immun. 1993;7:36–46. doi: 10.1006/brbi.1993.1004. [DOI] [PubMed] [Google Scholar]
- 62.Persoons J H A, Berkenbosch F, Schornagel K, Thepen T, Kraal G. J Allergy Clin Immunol. 1995;95:765–770. doi: 10.1016/s0091-6749(95)70184-2. [DOI] [PubMed] [Google Scholar]
- 63.Cohen J J. In: Anti-Inflammatory Steroid Action: Basic and Clinical Aspects. Schleimer R P, Claman H N, Oronsky A, editors. San Diego: Academic; 1989. pp. 110–131. [Google Scholar]
- 64.Haynes R C J. In: The Pharmacological Basis of Experimental Therapeutics. Gilman A G, Rall T W, Nies A S, Taylor P, editors. New York: Pergamon; 1990. pp. 1431–1462. [Google Scholar]
- 65.Hoffman B B, Lefkowitz R J. In: The Pharmacological Basis of Experimental Therapeutics. Gilman A G, Rall T W, Nies A S, Taylor P, editors. New York: Pergamon; 1990. pp. 187–241. [Google Scholar]