Abstract
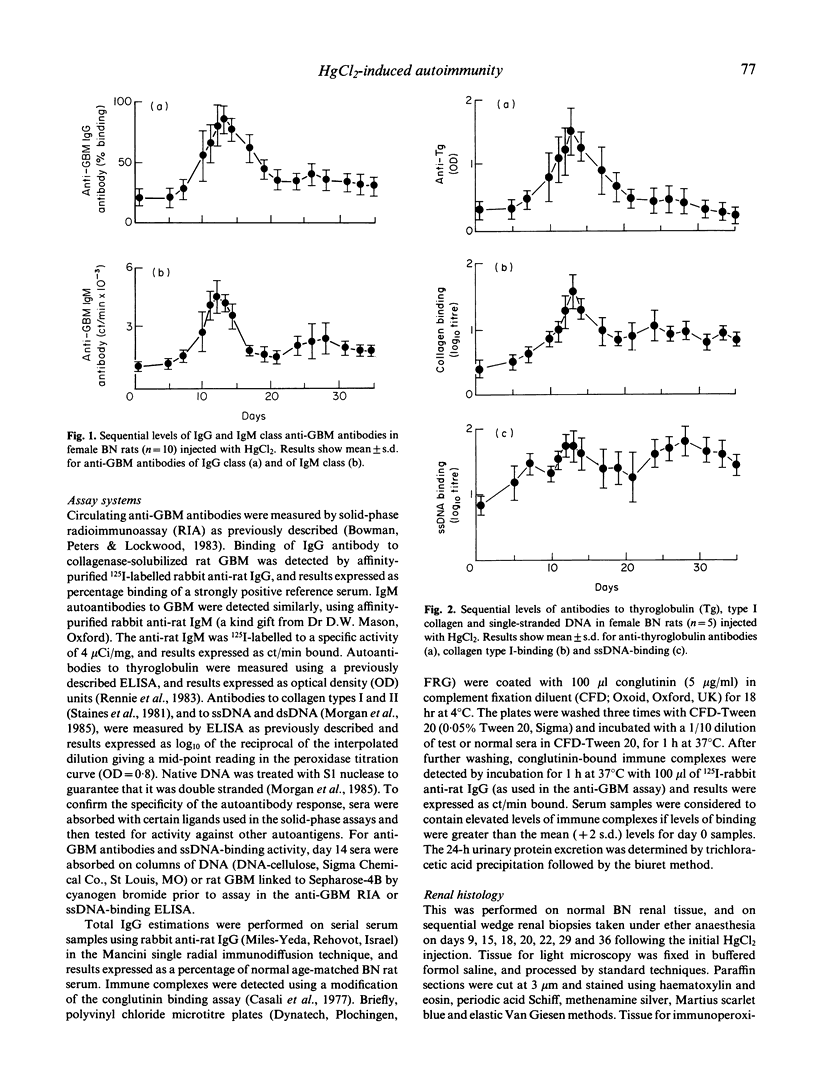
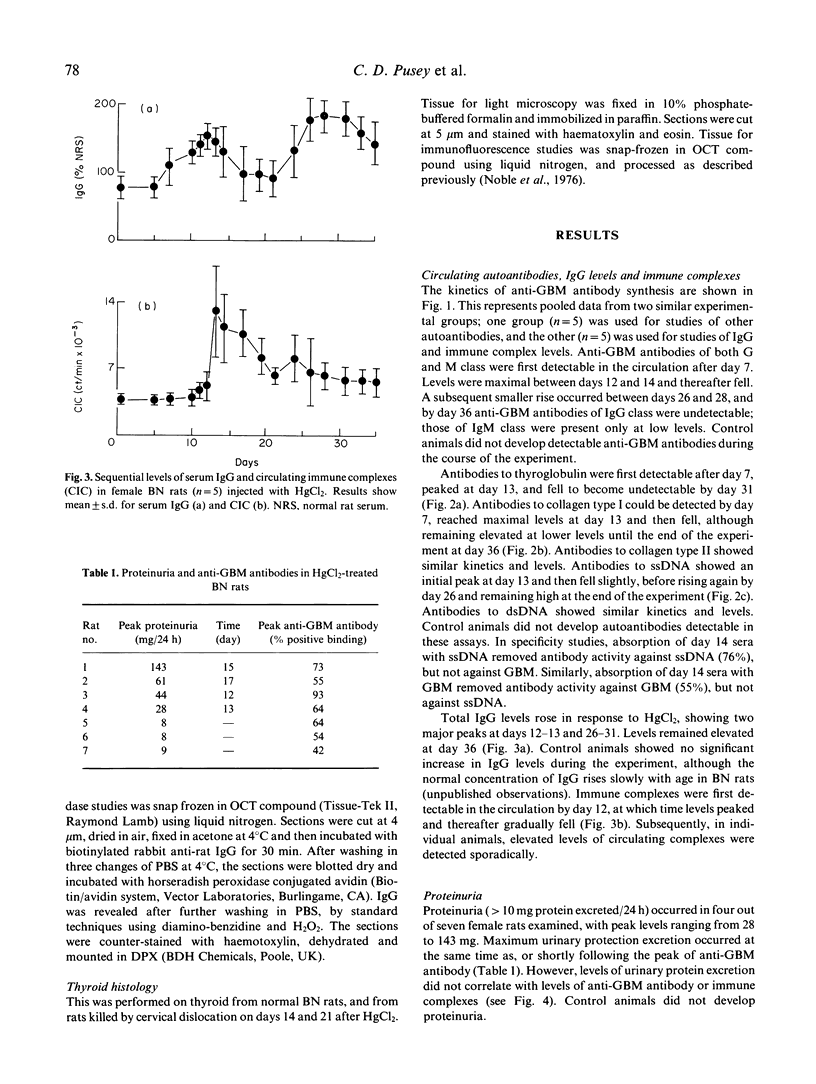
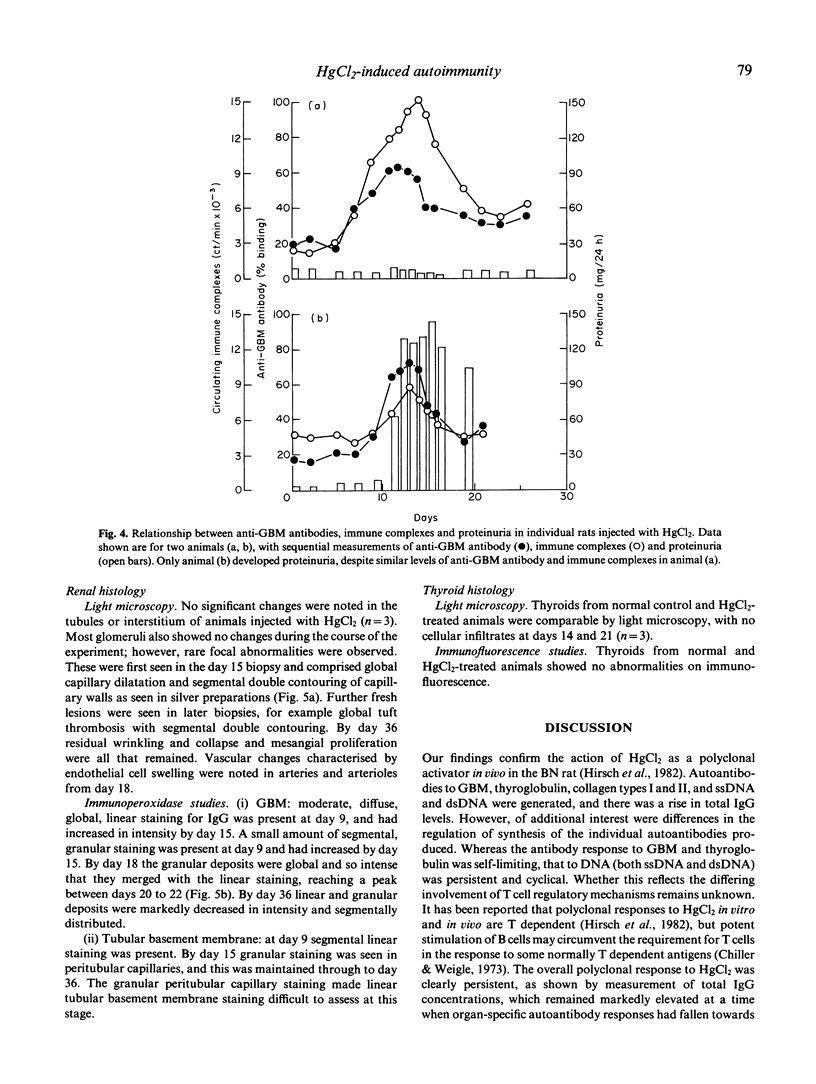
Repeated low-dose injections of mercuric chloride (HgCl2) in the brown Norway (BN) rat result in polyclonal activation which includes the induction of anti-glomerular basement membrane (GBM) autoantibodies. We examined the kinetics of various autoantibodies produced in vivo, general features of polyclonal activation such as total IgG levels and immune complex formation, and the relationship between organ specific autoimmunity and tissue injury in the kidney and thyroid. The production of immune complexes and autoantibodies to GBM and thyroglobulin was short lived, and the increase in levels of total IgG and antibodies to ssDNA and dsDNA was prolonged; the antibody response to collagen types I and II was intermediate in duration. Autoantibodies induced by HgCl2 caused only mild and variable tissue injury in the kidneys and did not induce abnormalities in the thyroid. These studies demonstrate that immunostimulation by mercury may result in the formation of a range of autoantibodies, with variable kinetics and pathogenicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellon B., Capron M., Druet E., Verroust P., Vial M. C., Sapin C., Girard J. F., Foidart J. M., Mahieu P., Druet P. Mercuric chloride induced autoimmune disease in Brown-Norway rats: sequential search for anti-basement membrane antibodies and circulating immune complexes. Eur J Clin Invest. 1982 Apr;12(2):127–133. doi: 10.1111/j.1365-2362.1982.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Bluestein H. G., Zvaifler N. J., Weisman M. H., Shapiro R. F. Lymphocyte alteration by procainamide: relation to drug-induced lupus erythematosus syndrome. Lancet. 1979 Oct 20;2(8147):816–819. doi: 10.1016/s0140-6736(79)92174-3. [DOI] [PubMed] [Google Scholar]
- Bowman C., Green C., Borysiewicz L., Lockwood C. M. Circulating T-cell populations during mercuric chloride-induced nephritis in the Brown Norway rat. Immunology. 1987 Aug;61(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Bowman C., Mason D. W., Pusey C. D., Lockwood C. M. Autoregulation of autoantibody synthesis in mercuric chloride nephritis in the Brown Norway rat. I. A role for T suppressor cells. Eur J Immunol. 1984 May;14(5):464–470. doi: 10.1002/eji.1830140515. [DOI] [PubMed] [Google Scholar]
- Bowman C., Peters D. K., Lockwood C. M. Anti-glomerular basement membrane autoantibodies in the Brown Norway rat: detection by a solid-phase radioimmunoassay. J Immunol Methods. 1983 Jul 29;61(3):325–333. doi: 10.1016/0022-1759(83)90227-2. [DOI] [PubMed] [Google Scholar]
- Camussi G., Brentjens J. R., Noble B., Kerjaschki D., Malavasi F., Roholt O. A., Farquhar M. G., Andres G. Antibody-induced redistribution of Heymann antigen on the surface of cultured glomerular visceral epithelial cells: possible role in the pathogenesis of Heymann glomerulonephritis. J Immunol. 1985 Oct;135(4):2409–2416. [PubMed] [Google Scholar]
- Casali P., Bossus A., Carpentier N. A., Lambert P. H. Solid-phase enzyme immunoassay or radioimmunoassay for the detection of immune complexes based on their recognition by conglutinin: conglutinin-binding test. A comparative study with 125I-labelled Clq binding and Raji-cell RIA tests. Clin Exp Immunol. 1977 Aug;29(2):342–354. [PMC free article] [PubMed] [Google Scholar]
- Ceuppens J. L., Goodwin J. S. Endogenous prostaglandin E2 enhances polyclonal immunoglobulin production by tonically inhibiting T suppressor cell activity. Cell Immunol. 1982 Jun;70(1):41–54. doi: 10.1016/0008-8749(82)90131-9. [DOI] [PubMed] [Google Scholar]
- Chalopin J. M., Lockwood C. M. Autoregulation of autoantibody synthesis in mercuric chloride nephritis in the Brown Norway rat. II. Presence of antigen-augmentable plaque-forming cells in the spleen is associated with humoral factors behaving as auto-anti-idiotypic antibodies. Eur J Immunol. 1984 May;14(5):470–475. doi: 10.1002/eji.1830140516. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Weigle W. O. Termination of tolerance to human gamma globulin in mice by antigen and bacterial lipopolysaccharide (endotoxin). J Exp Med. 1973 Mar 1;137(3):740–750. doi: 10.1084/jem.137.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker A. J., Venuto R. C., Vladutiu A. O., Brentjens J. R., Andres G. A. Effects of prolonged administration of D-penicillamine or captopril in various strains of rats. Brown Norway rats treated with D-penicillamine develop autoantibodies, circulating immune complexes, and disseminated intravascular coagulation. Clin Immunol Immunopathol. 1984 Jan;30(1):142–155. doi: 10.1016/0090-1229(84)90015-1. [DOI] [PubMed] [Google Scholar]
- Druet P., Ayed K., Bariety J., Bernaudin J. F., Druet E., Girard J. F., Hinglais N., Sapin C. Experimental immune glomerulonephritis induced in the rat by mercuric chloride. Adv Nephrol Necker Hosp. 1979;8:321–342. [PubMed] [Google Scholar]
- Fauci A. S., Lane H. C., Volkman D. J. Activation and regulation of human immune responses: implications in normal and disease states. Ann Intern Med. 1983 Jul;99(1):61–75. doi: 10.7326/0003-4819-99-1-61. [DOI] [PubMed] [Google Scholar]
- Hinglais N., Druet P., Grossetete J., Sapin C., Bariety J. Ultrastructural study of nephritis induced in Brown Norway rats by mercuric chloride. Lab Invest. 1979 Aug;41(2):150–159. [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Jaffe I. A. Induction of auto-immune syndromes by penicillamine therapy in rheumatoid arthritis and other diseases. Springer Semin Immunopathol. 1981;4(2):193–207. doi: 10.1007/BF01857095. [DOI] [PubMed] [Google Scholar]
- Kirtland H. H., 3rd, Mohler D. N., Horwitz D. A. Methyldopa inhibition of suppressor-lymphocyte function: a proposed cause of autoimmune hemolytic anemia. N Engl J Med. 1980 Apr 10;302(15):825–832. doi: 10.1056/NEJM198004103021502. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S., Rose N. R. Humoral and cellular immune response to thyroglobulin in different inbred rat strains. Clin Exp Immunol. 1982 Mar;47(3):661–669. [PMC free article] [PubMed] [Google Scholar]
- Michaud A., Sapin C., Leca G., Aiach M., Druet P. Involvement of hemostasis during an autoimmune glomerulonephritis induced by mercuric chloride in brown Norway rats. Thromb Res. 1984 Jan 1;33(1):77–88. doi: 10.1016/0049-3848(84)90156-7. [DOI] [PubMed] [Google Scholar]
- Morgan A., Buchanan R. R., Lew A. M., Olsen I., Staines N. A. Five groups of antigenic determinants on DNA identified by monoclonal antibodies from (NZB X NZW)F1 and MRL/Mp-lpr/lpr mice. Immunology. 1985 May;55(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Noble B., Yoshida T., Rose N. R., Bigazzi P. E. Thyroid antibodies in spontaneous autoimmune thyroiditis in the Buffalo rat. J Immunol. 1976 Nov;117(5 Pt 1):1447–1455. [PubMed] [Google Scholar]
- Ochi T., Goldings E. A., Lipsky P. E., Ziff M. Immunomodulatory effect of procainamide in man. Inhibition of human suppressor T-cell activity in vitro. J Clin Invest. 1983 Jan;71(1):36–45. doi: 10.1172/JCI110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Hirsch F., Sapin C., Druet P. Autoreactive T cells in mercury-induced autoimmune disease: in vitro demonstration. J Immunol. 1986 Oct 15;137(8):2548–2554. [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Hirsch F., Sapin C., Druet P. In vivo self-reactivity of mononuclear cells to T cells and macrophages exposed to HgCl2. Eur J Immunol. 1985 May;15(5):460–465. doi: 10.1002/eji.1830150509. [DOI] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Rossert J., Vial M. C., Mandet C., Druet P. Autoreactive T cells in mercury-induced autoimmunity. Ability to induce the autoimmune disease. J Immunol. 1988 Feb 1;140(3):750–754. [PubMed] [Google Scholar]
- Rees A. J., Lockwood C. M., Peters D. K. Enhanced allergic tissue injury in Goodpasture's syndrome by intercurrent bacterial infection. Br Med J. 1977 Sep 17;2(6089):723–726. doi: 10.1136/bmj.2.6089.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie D. P., McGregor A. M., Keast D., Weetman A. P., Foord S. M., Dieguez C., Williams E. D., Hall R. The influence of methimazole on thyroglobulin-induced autoimmune thyroiditis in the rat. Endocrinology. 1983 Jan;112(1):326–330. doi: 10.1210/endo-112-1-326. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Reimer G., McNally E. M., Nusinow S. R., Searles R. P., Tan E. M. Procainamide elicits a selective autoantibody immune response. Clin Exp Immunol. 1986 Jan;63(1):58–67. [PMC free article] [PubMed] [Google Scholar]
- Staines N. A., Hardingham T., Smith M., Henderson B. Collagen-induced arthritis in the rat: modification of immune and arthritic responses by free collagen and immune anti-collagen antiserum. Immunology. 1981 Dec;44(4):737–744. [PMC free article] [PubMed] [Google Scholar]
- Tipping P. G., Neale T. J., Holdsworth S. R. T lymphocyte participation in antibody-induced experimental glomerulonephritis. Kidney Int. 1985 Mar;27(3):530–537. doi: 10.1038/ki.1985.43. [DOI] [PubMed] [Google Scholar]
- Tomosugi N. I., Cashman S. J., Hay H., Pusey C. D., Evans D. J., Shaw A., Rees A. J. Modulation of antibody-mediated glomerular injury in vivo by bacterial lipopolysaccharide, tumor necrosis factor, and IL-1. J Immunol. 1989 May 1;142(9):3083–3090. [PubMed] [Google Scholar]
- Wedeen R. P. Occupational renal disease. Am J Kidney Dis. 1984 Jan;3(4):241–257. doi: 10.1016/s0272-6386(84)80041-4. [DOI] [PubMed] [Google Scholar]
- Weening J. J., Hoedemaeker P. J., Bakker W. W. Immunoregulation and anti-nuclear antibodies in mercury-induced glomerulopathy in the rat. Clin Exp Immunol. 1981 Jul;45(1):64–71. [PMC free article] [PubMed] [Google Scholar]



