Abstract
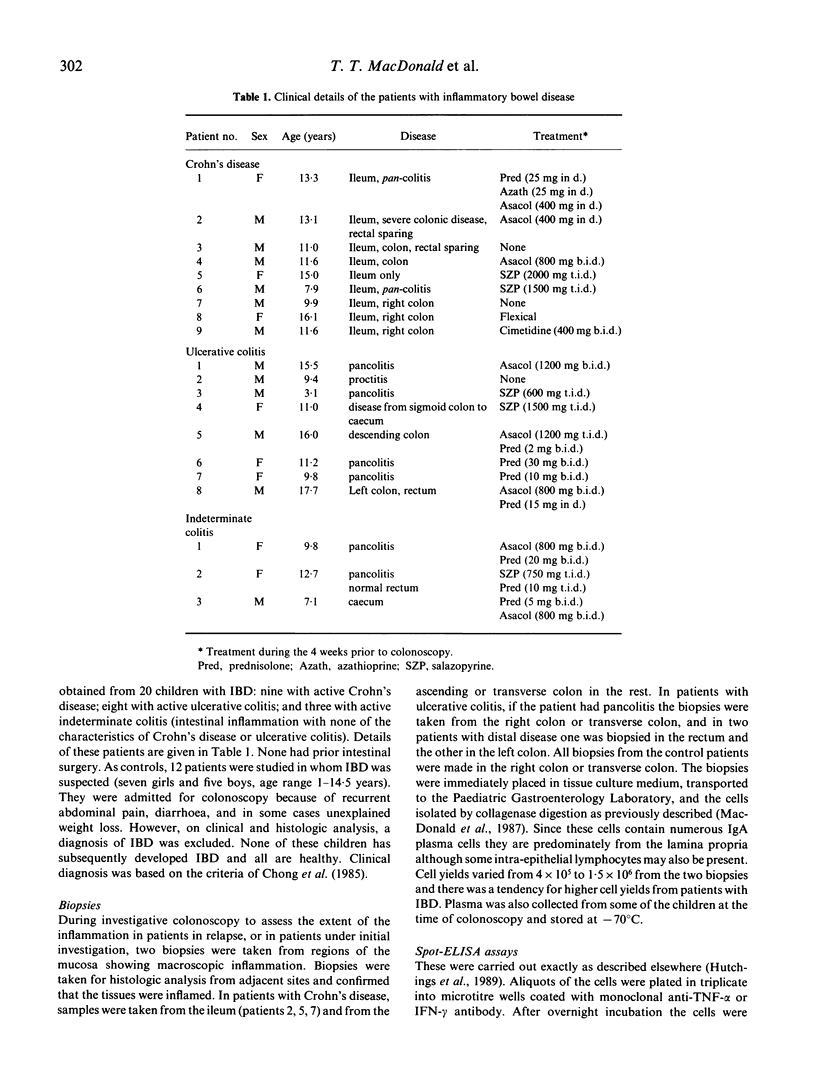
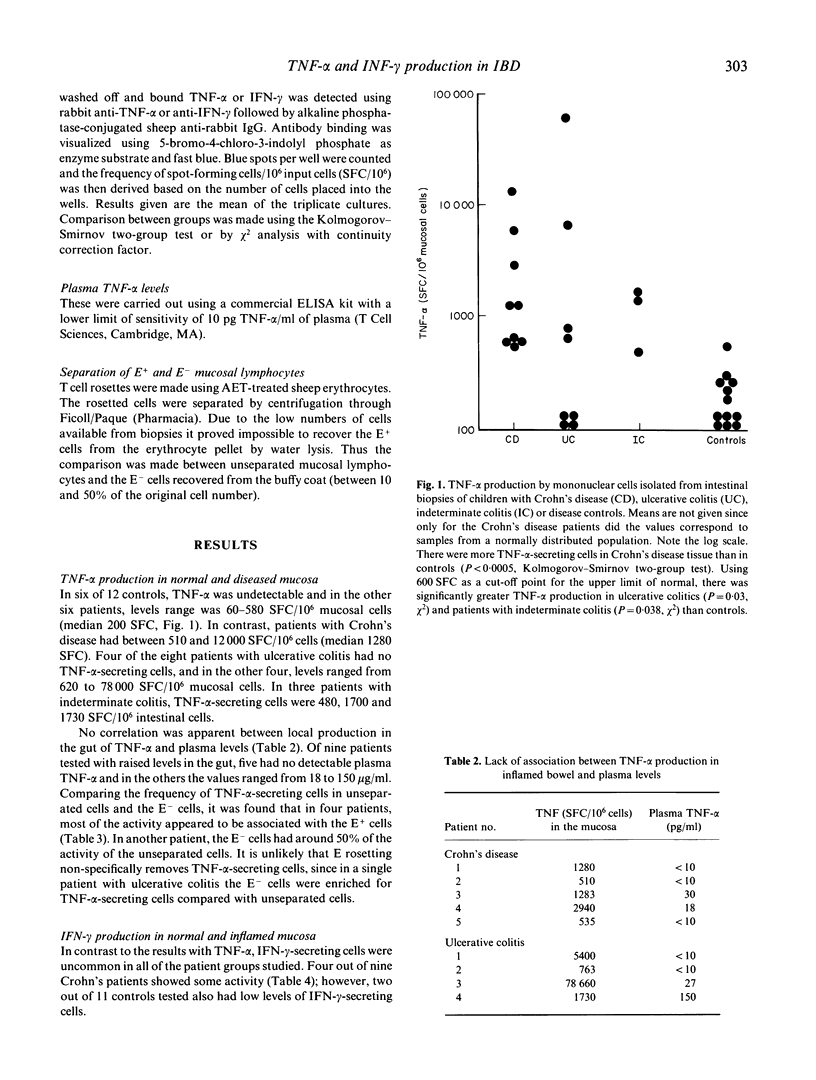
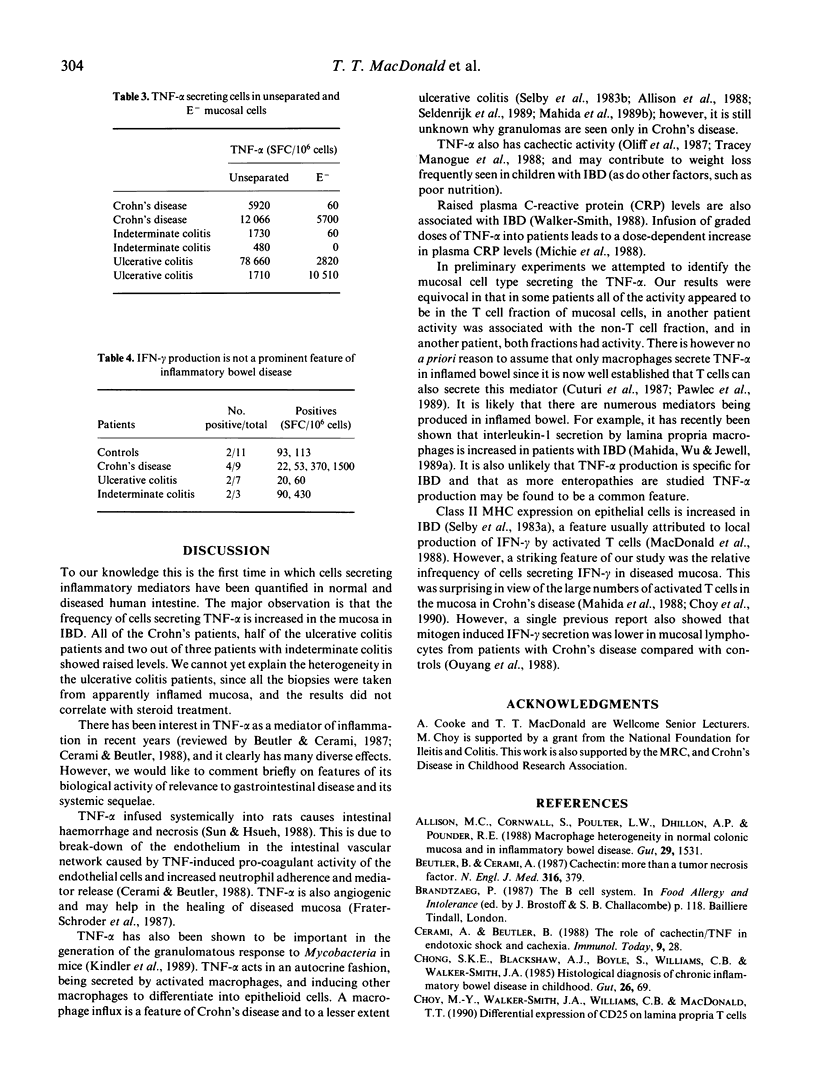
The spot-ELISA technique has been used to enumerate the frequency of cells secreting tumor necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma), isolated from biopsies of normal intestine and from biopsies of children with inflammatory bowel disease. TNF-alpha production was undetectable in six out of 12 biopsies from normal intestine and in the other six biopsies it ranged from 60 to 580 TNF-alpha-secreting cells/10(6) isolated intestinal cells. In contrast, cells isolated from biopsies of children with Crohn's disease (n = 9) all showed elevated frequencies of TNF-alpha-secreting cells (500-12,000 secreting cells/10(6) cells). In ulcerative colitis, four out of eight children had increased production of TNF-alpha and in children with indeterminate colitis two out of three had elevated levels. There was no correlation between plasma TNF-alpha levels and the number of intestinal cells secreting TNF-alpha. In controls and all groups of patients IFN-gamma-secreting cells were uncommon. These results suggest that TNF-alpha is an important mediator of inflammation in the human gut, and, furthermore, may play a role in the growth failure frequently seen in children with inflammatory bowel disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Cornwall S., Poulter L. W., Dhillon A. P., Pounder R. E. Macrophage heterogeneity in normal colonic mucosa and in inflammatory bowel disease. Gut. 1988 Nov;29(11):1531–1538. doi: 10.1136/gut.29.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Machelski E., Weiserbs D. B. T cell-B cell regulation in the intestinal lamina propria in Crohn's disease. Gastroenterology. 1985 Aug;89(2):321–327. doi: 10.1016/0016-5085(85)90332-4. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Youngman K. R., Farmer R. G. Immunoregulatory function of human intestinal mucosa lymphoid cells: evidence for enhanced suppressor cell activity in inflammatory bowel disease. Gut. 1983 Aug;24(8):692–701. doi: 10.1136/gut.24.8.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings P. R., Cambridge G., Tite J. P., Meager T., Cooke A. The detection and enumeration of cytokine-secreting cells in mice and man and the clinical application of these assays. J Immunol Methods. 1989 Jun 2;120(1):1–8. doi: 10.1016/0022-1759(89)90281-0. [DOI] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Immunoregulatory function of lamina propria T cells in Crohn's disease. Gastroenterology. 1985 May;88(5 Pt 1):1143–1150. doi: 10.1016/s0016-5085(85)80073-1. [DOI] [PubMed] [Google Scholar]
- LOCKHART-MUMMERY H. E., MORSON B. C. Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut. 1960 Jun;1:87–105. doi: 10.1136/gut.1.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. In vivo profiles of eicosanoids in ulcerative colitis, Crohn's colitis, and Clostridium difficile colitis. Gastroenterology. 1988 Jul;95(1):11–17. doi: 10.1016/0016-5085(88)90284-3. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Mohrman R. F., Kodner I. J., Delacroix D. L., Vaerman J. P. Altered patterns of secretion of monomeric IgA and IgA subclass 1 by intestinal mononuclear cells in inflammatory bowel disease. Gastroenterology. 1986 Aug;91(2):379–385. doi: 10.1016/0016-5085(86)90572-x. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Weinel A., Spencer J. HLA-DR expression in human fetal intestinal epithelium. Gut. 1988 Oct;29(10):1342–1348. doi: 10.1136/gut.29.10.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Gionchetti P., Vaux D., Jewell D. P. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989 Jun;30(6):826–834. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Wu K., Jewell D. P. Interleukin 2 receptor expression by macrophages in inflammatory bowel disease. Clin Exp Immunol. 1988 Dec;74(3):382–386. [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. F., Eastwood M. A., Brydon W. G. Methane excretion in man--a study of breath, flatus, and faeces. Gut. 1985 Jan;26(1):69–74. doi: 10.1136/gut.26.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Spriggs D. R., Manogue K. R., Sherman M. L., Revhaug A., O'Dwyer S. T., Arthur K., Dinarello C. A., Cerami A., Wolff S. M. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery. 1988 Aug;104(2):280–286. [PubMed] [Google Scholar]
- O'Donoghue D. P., Dawson A. M. Crohn's disease in childhood. Arch Dis Child. 1977 Aug;52(8):627–632. doi: 10.1136/adc.52.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Pawelec G., Schaudt K., Rehbein A., Busch F. W. Differential secretion of tumor necrosis factor-alpha and granulocyte/macrophage colony-stimulating factors but not interferon-gamma from CD4+ compared to CD8+ human T cell clones. Eur J Immunol. 1989 Jan;19(1):197–200. doi: 10.1002/eji.1830190132. [DOI] [PubMed] [Google Scholar]
- Qin O. Y., el-Youssef M., Yen-Lieberman B., Sapatnekar W., Youngman K. R., Kusugami K., Fiocchi C. Expression of HLA-DR antigens in inflammatory bowel disease mucosa: role of intestinal lamina propria mononuclear cell-derived interferon gamma. Dig Dis Sci. 1988 Dec;33(12):1528–1536. doi: 10.1007/BF01535942. [DOI] [PubMed] [Google Scholar]
- RAPPAPORT H., BURGOYNE F. H., SMETANA H. F. The pathology of regional enteritis. Mil Surg. 1951 Oct;109(4):463–502. [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Mason D. Y., Jewell D. P. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol. 1983 Sep;53(3):614–618. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Poulter L. W., Hobbs S., Jewell D. P., Janossy G. Heterogeneity of HLA-DR-positive histiocytes in human intestinal lamina propria: a combined histochemical and immunohistological analysis. J Clin Pathol. 1983 Apr;36(4):379–384. doi: 10.1136/jcp.36.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldenrijk C. A., Drexhage H. A., Meuwissen S. G., Pals S. T., Meijer C. J. Dendritic cells and scavenger macrophages in chronic inflammatory bowel disease. Gut. 1989 Apr;30(4):484–491. doi: 10.1136/gut.30.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. M., Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988 May;81(5):1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifroni A., Treves A. J., Sachar D. B., Rachmilewitz D. Prostanoid synthesis by cultured intestinal epithelial and mononuclear cells in inflammatory bowel disease. Gut. 1983 Jul;24(7):659–664. doi: 10.1136/gut.24.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]


