Abstract
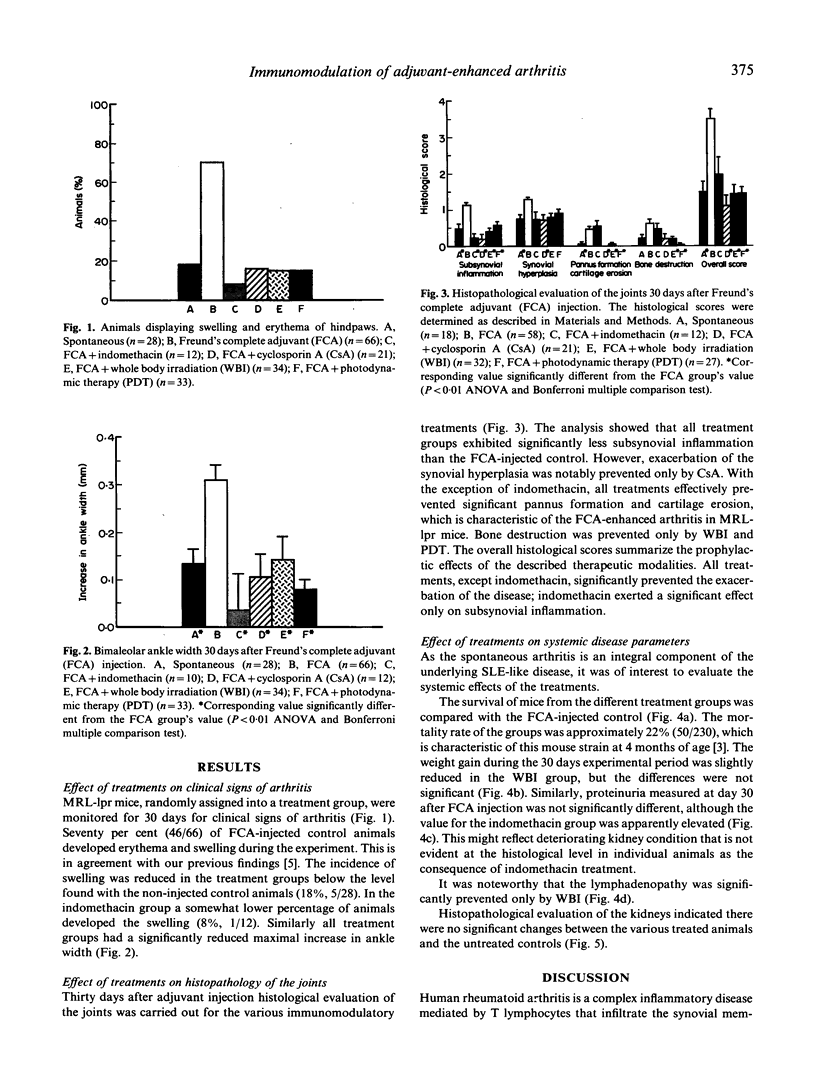
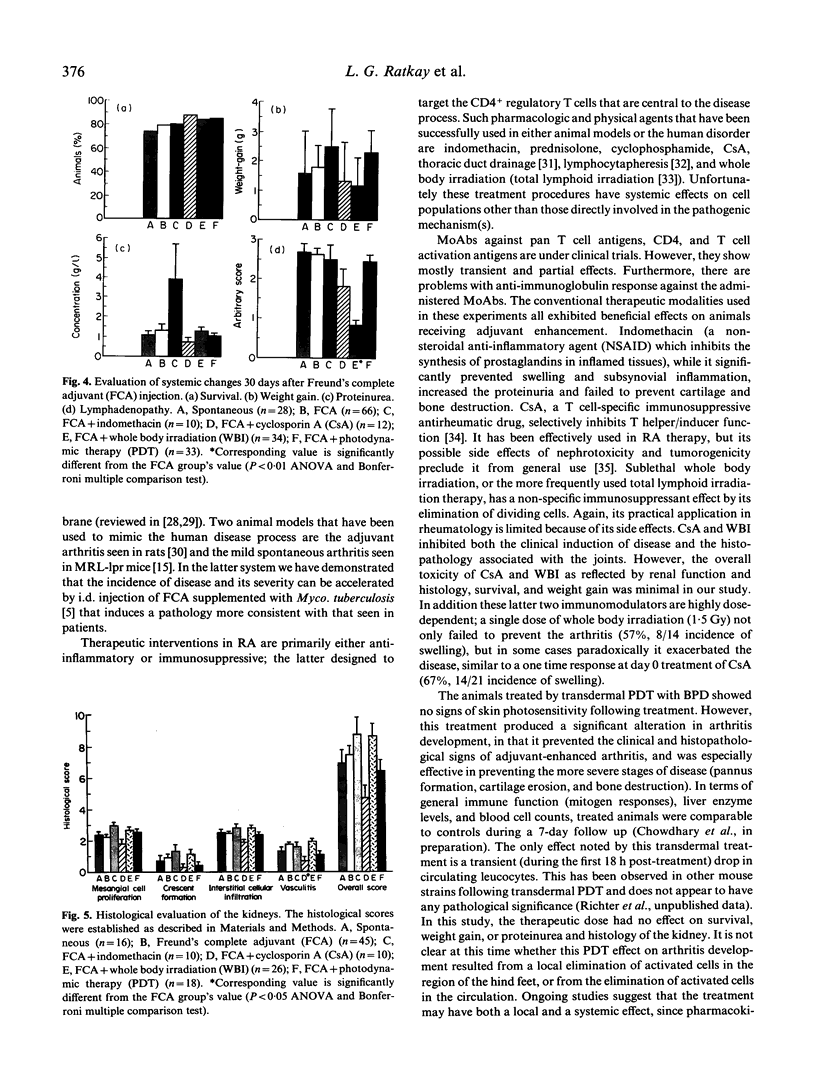
Although numerous experimental immunomodulatory regimens have been reported to be effective in the treatment of rheumatoid arthritis, they also produce undesirable side effects. An alternative specific modality of localized treatment is photodynamic therapy (PDT). In this study we treated 13-week-old MRL-lpr mice whose spontaneous arthritis was enhanced by intradermal injection of Freund's complete adjuvant (FCA). One group received transcutaneous photodynamic therapy at days 0, 10, and 20, following the FCA injection. The other groups were injected with 1 mg/kg per day indomethacin, 40 mg/kg per day cyclosporin A (CsA), or treated with 3 Gy sublethal whole body irradiation (WBI). The development of swelling was monitored for 1 month, at which time proteinuria, lymphadenopathy and the histopathology of the joints and kidneys were assessed. The results demonstrated that PDT and the conventional treatments significantly ameliorated swelling of the hindlimbs from 70% in the untreated FCA-injected animals to below the 19% level characteristic of the unmanipulated control. Histological examination showed a reduction in pannus formation, and cartilage and bone destruction, the characteristics of adjuvant-enhanced arthritis. PDT did not affect the survival rate, lymphoproliferation, or proteinuria of the treated animals. However, indomethacin increased proteinuria, and was less effective in preventing cartilage and bone destruction. Furthermore, lower doses of CsA and WBI exacerbated arthritis activity. These results indicate that photodynamic therapy can inhibit the development of adjuvant-enhanced arthritis in MRL-lpr mice with similar effectiveness to the conventional treatments, but without their negative side effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden J. H., Faaber P., Assmann K. J., Rijke T. P. Effects of cyclosporin A on autoimmune disease in MRL/1 and BXSB mice. Scand J Immunol. 1986 Oct;24(4):405–411. doi: 10.1111/j.1365-3083.1986.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Berger C. L., Perez M., Laroche L., Edelson R. Inhibition of autoimmune disease in a murine model of systemic lupus erythematosus induced by exposure to syngeneic photoinactivated lymphocytes. J Invest Dermatol. 1990 Jan;94(1):52–57. doi: 10.1111/1523-1747.ep12873349. [DOI] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 29-1989. A 79-year-old man with fever, abdominal pain, and inflamed right eye. N Engl J Med. 1989 Jul 20;321(3):172–182. doi: 10.1056/NEJM198907203210307. [DOI] [PubMed] [Google Scholar]
- Dixon F. J. Basic elements of murine systemic lupus erythematosus. J Rheumatol Suppl. 1987 Jun;14 (Suppl 13):3–10. [PubMed] [Google Scholar]
- Emery P., Smith G. N., Panayi G. S. Lymphocytapheresis--a feasible treatment for rheumatoid arthritis. Br J Rheumatol. 1986 Feb;25(1):40–43. doi: 10.1093/rheumatology/25.1.40. [DOI] [PubMed] [Google Scholar]
- Gilkeson G. S., Spurney R., Coffman T. M., Kurlander R., Ruiz P., Pisetsky D. S. Effect of anti-CD4 antibody treatment on inflammatory arthritis in MRL-lpr/lpr mice. Clin Immunol Immunopathol. 1992 Aug;64(2):166–172. doi: 10.1016/0090-1229(92)90195-t. [DOI] [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Dixon F. J. A spontaneous rheumatoid arthritis-like disease in MRL/l mice. J Exp Med. 1982 Jun 1;155(6):1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Kingsley G., Pitzalis C., Panayi G. S. Immunogenetic and cellular immune mechanisms in rheumatoid arthritis: relevance to new therapeutic strategies. Br J Rheumatol. 1990 Feb;29(1):58–64. doi: 10.1093/rheumatology/29.1.58. [DOI] [PubMed] [Google Scholar]
- McCune W. J., Bayliss G. E. Immunosuppressive drug therapy for rheumatic disease. Curr Opin Rheumatol. 1991 Jun;3(3):355–362. doi: 10.1097/00002281-199106000-00005. [DOI] [PubMed] [Google Scholar]
- Mountz J. D., Smith H. R., Wilder R. L., Reeves J. P., Steinberg A. D. CS-A therapy in MRL-lpr/lpr mice: amelioration of immunopathology despite autoantibody production. J Immunol. 1987 Jan 1;138(1):157–163. [PubMed] [Google Scholar]
- North J., Neyndorff H., Levy J. G. Photosensitizers as virucidal agents. J Photochem Photobiol B. 1993 Feb;17(2):99–108. doi: 10.1016/1011-1344(93)80002-q. [DOI] [PubMed] [Google Scholar]
- O'Sullivan F. X., Ray C. J., Takeda Y., Sharp G. C., Walker S. E. Long-term anti-CD4 treatment of MRL/lpr mice ameliorates immunopathology and lymphoproliferation but fails to suppress rheumatoid factor production. Clin Immunol Immunopathol. 1991 Dec;61(3):421–435. doi: 10.1016/s0090-1229(05)80013-3. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- Paulus H. E., Machleder H. I., Levine S., Yu D. T., MacDonald N. S. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977 Jul-Aug;20(6):1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- Ratkay L. G., Tonzetich J., Waterfield J. D. Antibodies to extracellular matrix proteins in the sera of MRL-lpr mice. Clin Immunol Immunopathol. 1991 May;59(2):236–245. doi: 10.1016/0090-1229(91)90021-2. [DOI] [PubMed] [Google Scholar]
- Ratkay L. G., Zhang L., Tonzetich J., Waterfield J. D. Complete Freund's adjuvant induces an earlier and more severe arthritis in MRL-lpr mice. J Immunol. 1993 Nov 1;151(9):5081–5087. [PubMed] [Google Scholar]
- Richter A. M., Waterfield E., Jain A. K., Sternberg E. D., Dolphin D., Levy J. G. In vitro evaluation of phototoxic properties of four structurally related benzoporphyrin derivatives. Photochem Photobiol. 1990 Sep;52(3):495–500. doi: 10.1111/j.1751-1097.1990.tb01791.x. [DOI] [PubMed] [Google Scholar]
- Richter A. M., Yip S., Waterfield E., Logan P. M., Slonecker C. E., Levy J. G. Mouse skin photosensitization with benzoporphyrin derivatives and Photofrin: macroscopic and microscopic evaluation. Photochem Photobiol. 1991 Feb;53(2):281–286. doi: 10.1111/j.1751-1097.1991.tb03935.x. [DOI] [PubMed] [Google Scholar]
- Rordorf-Adam C., Rordorf B., Serban D., Pataki A. The effects of anti-inflammatory agents on the serology and arthritis of the MRL lpr/lpr mouse. Agents Actions. 1986 Dec;19(5-6):309–310. doi: 10.1007/BF01971233. [DOI] [PubMed] [Google Scholar]
- Rordorf C., Pataki A., Nogues V., Schlager F., Feige U., Glatt M. Arthritis in MRL/LPR mice and in collagen II sensitized DBA-1 mice and their use in pharmacology. Int J Tissue React. 1987;9(4):341–347. [PubMed] [Google Scholar]
- Santoro T. J., Lehmann K. R., Batt R. A., Kotzin B. L. The role of L3T4+ cells in the pathogenesis of lupus in lpr-bearing mice. I. Defects in the production of interleukins 2 and 3. Eur J Immunol. 1987 Aug;17(8):1131–1136. doi: 10.1002/eji.1830170809. [DOI] [PubMed] [Google Scholar]
- Santoro T. J., Portanova J. P., Kotzin B. L. The contribution of L3T4+ T cells to lymphoproliferation and autoantibody production in MRL-lpr/lpr mice. J Exp Med. 1988 May 1;167(5):1713–1718. doi: 10.1084/jem.167.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman W. E., Wofsy D., Greenspan J. S., Ledbetter J. A. Treatment of autoimmune MRL/Ipr mice with monoclonal antibody to Thy-1.2: a single injection has sustained effects on lymphoproliferation and renal disease. J Immunol. 1983 Apr;130(4):1713–1718. [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Balderas R., Shawler D. L., Izui S., Kotzin B. L., Strober S., Dixon F. J. Inhibition of T cells proliferation and SLE-like syndrome of MRL/1 mice by whole body or total lymphoid irradiation. J Immunol. 1980 Nov;125(5):2137–2142. [PubMed] [Google Scholar]
- Wofsy D., Ledbetter J. A., Hendler P. L., Seaman W. E. Treatment of murine lupus with monoclonal anti-T cell antibody. J Immunol. 1985 Feb;134(2):852–857. [PubMed] [Google Scholar]


